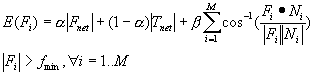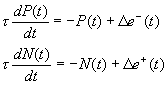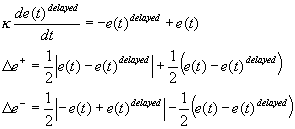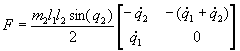ACKNOWLEDGEMENTS
The
time I had at USC during the Ph.D. route was a very enriching period of my
life. I had the opportunity to work in an exciting and stimulating research
environment, led by Michael Arbib.
I
would like to present my deepest gratitude to The Scientific and Technical
Research Council of Turkey (TUBITAK) for providing me the scholarship that made
it possible for me to attempt and complete the Ph.D. study presented in this
thesis. The study would not have been possible if TUBITAK did not provide
support for the very first semester and the final semester at USC.
I
would like to thank Michael Arbib for guiding and educating me throughout the
my years at USC. He has been an extraordinary advisor and mentor, whom I owe
all the brain theory I learned. I would also like to present my
gratitude to Michael Arbib for providing support via HFSP and USCBP grants.
Without HFSP and USCBP support, this study would not be possible.
Stefan
Schaal is a great mentor, who has been a constant support and source of
inspiration with never-ending energy. Besides introducing me to Robotics, he
and his colleague, Sethu Vijayakumar were very influential in maturating the
concept of machine learning in my mind.
I
also owe a great debt of gratitude to Nina Bradley for being a source positive
energy and mentoring me especially in infant motor development. Without
her, the thesis would be lacking a major component.
I
am also full of gratitude to my Ph.D. qualification exam comittee members Maja
Mataric and Christoph von der Malsburg for their guidance and support.
I
am greatly indebted to Giacomo Rizzolatti
for enabling me to visit his lab and providing the opportunity to
communicate with not only himself but
also with Vittorio Gallese and
Leonardo Fogassi who have provided
invaluable
insights about mirror neurons. In addition, I am very thankful to
Massimo Matelli
and Giuseppe Luppino,
for the first hand information on the
mirror neuron system connectivity, and to Luciano Fadiga for stimulating
discussions. I would also like to thank to Christian Keysers and Evelyne
(Kohler) for not only actively involving me in their recording sessions but
also offering their sincere friendship.
I
am very thankful to Hideo Sakata for giving me the opportunity to visit his lab
in Tokyo and interact with many researchers including Murata-san with whom I
had very stimulating discussions.
I
am deeply thankful to Mitsuo Kawato, for giving me the opportunity to interact
with various researchers in Japan by having me at ATR during the summer of
2001. My research experience at ATR was very rewarding; I greatly expanded my
knowledge on motor control and motor learning. I would like to salute the staff
at ATR for all their help. I also would like to present my thanks to the
friends at ATR for welcoming me and making me feel at home.
I
would like to present my appreciation and thanks to my mentors at Middle East
Technical University in Turkey. I present my sincere thanks to my masters
advisor Marifi Guler for introducing me to neural computation and to Volkan
Atalay for introducing me to computer vision, and supporting my Ph.D. application.
Especially, I would like to present my gratitude to Fatos Yarman Vural for her
guidance and support during my Masters study and for preparing me for the Ph.D.
work presented in this dissertation. Other influential Computer Science
professors to whom I am grateful for educating me are Nese Yalabik, Gokturk
Ucoluk and Sinan Neftci.
I
would like to present my gratitude to Tosun Terzioglu, Albert Ekip, Turgut
Onder and Semih Koray who were professors of the Mathematics Department at the
Middle East Technical University. They taught me how to think ‘right’ and
exposed the beauty of mathematics to me.
Throughout
these six years at USC, I had the pleasure to meet several valuable people who
contributed to this dissertation. Firstly, I am very thankful to Aude Billard
and Auke Jan Ijspeert for all their support and scientific interaction and
feedback. They have a huge role in helping me get through the tough times
during my Ph.D. work. In addition, I would like to thank Aude Billard for
providing me the computing hardware and helping me have a nice working
environment, which was very essential for the progress of my Ph.D. study. I am
thankful to my great friend Sethu Vijayakumar for his support and stimulating
discussions. Jun Nakanishi, Jun Mei Zu, Aaron D’Souza, Jan Peters and Kazunori
Okada besides being of great support, were always there to discuss issues and
helped me overcome various obstacles. I am deeply thankful to Shrija Kumari for
offering not only her smile and friendship but also her energy to proofread my
manuscript. I owe a lot to Jun Mei Zu: she has always been there as a great
friend and has always offered her help and support when I needed it most. I am
indebted to my ex-officemate and a very valuable friend Mihail Bota for his
constant support and interactions for improving the thesis and providing me the
psychological support to overcome many obstacles throughout my Ph.D. years.
Finally, I would like to thank Juan Salvador Yahya Marmol for being a good
friend and sharing my workload during various periods of my Ph.D. I count
myself very lucky to have these great friends and colleagues whom once again I
present my gratitude: Thank you guys!
Not
a Hedco Neuroscience inhabitant but a very valuable friend, Lay Khim Ong was
always there for offering her help both psychologically and physically (Hey
Khim: thank you for your great editing!). I would like to thank other great
friends who supported me (in spite of my negligence in keeping in touch with
them). Kyle Reynolds, Awe Vinijkul, Aye
Vinijkul Reynolds, Alper Sen, Ebru Dincel: please accept my sincere thanks and
appreciation.
I
owe deep gratitude to my wife Mika Satomi for her patience in dealing with me
in difficult times. She was always
there. Her contribution to this thesis is indispensable. I especially celebrate
and thank her for the artistic talents and hard work that she generously
offered me throughout the Ph.D. study.
I
am greatly indebted to Paulina Tagle and Laura Lopez for their support and help
over all these years. I also would like to thank Laura Lopez and Yolanda Solis
for their kind friendship and support. My gratitude to Luigi Manna, who helped
me with the hardware and software issues during the Ph.D. study.
I
would like to thank Laurent Itti for his generous help for improving our lab
environment and providing partial support for my research. Also, I would like
to salute his lab members for their support and friendship. Florence Miau, in
particular, had always offered her warm friendship during her internship at
USC.
I
would like to present my appreciation to the good things in life particularly,
I would like to thank the ocean for comforting and rejuvenating me during
difficult times.
Finally,
I am deeply indebted to my family, to whom I owe much more than what can be
expressed. This work would not be possible without the help of my parents.
(Anne ve Baba, Evrim ve Nurdan: Hersey icin cok cok tesekkurler!)
LIST OF FIGURES
Figure 2.1 Lateral view of macaque brain
showing the areas of agranular frontal cortex and posterior parietal cortex
(adapted from Geyer et al. 2000). The naming conventions: frontal regions,
Matelli et al.(1991); parietal regions, Pandya and Seltzer (1982) 5
Figure
2.2 A canonical neuron response during grasping of various objects in the dark
(left to right and top to bottom: plate, ring, cube, cylinder, cone and sphere.
The rasters and histograms are aligned with object presentation. Small grey
bars in each raster marks onset of key press, go signal, key release, onset of
object pulling, release signal, and object release, respectively. The peaks in
ring and sphere object cases correspond to the grasping of the object by the
monkey (adapted from Murata et al. 1997a) 6
Figure
2.3 The motor responses of the same neuron shown in Figure 2.2. The motor
preference of the neuron is also carried over to the visual preference (compare
the ring and sphere histograms of both figures) (adapted from Murata et al.
1997a) 7
Figure
2.4 Activity of a cell during action observation (left) and action execution
(right). There is no activity in presentation of the object during both initial
presentation and bringing the tray towards the monkey. The vertical line over
the histogram indicates the hand-object contact onset. (from Gallese et al.,
1996). 8
Figure
2.5 Visual response of a mirror neuron. A. Precision grasp B. power grasp C.
mimicking of precision grasp. The vertical lines over the histograms indicate
the hand-object contact onset. (adapted from Gallese et al., 1996) 9
Figure 2.6 Example
of a strictly congruent manipulating mirror neuron: A) The experimenter
retrieved the food from a well in a tray. B) Same action, but performed by the
monkey. C) The monkey grasped a small piece of food using a precision grip. The
vertical lines over the histograms indicate the hand-object contact onset
(adapted from Gallese et al., 1996). 9
Figure 2.7 The
classification of area F5 neurons derived from published literature
(Dipellegrino et al. 1992; Gallese 2002; Gallese et al. 1996; Murata et al.
1997a; Murata et al. 1997b; Rizzolatti et al. 1996a; Rizzolatti and Gallese
2001). All F5 neurons fire in response to some motor action. In addition,
canonical neurons fire for object presentation while the mirror neurons fire
for action observation. The majority of hand related F5 neurons are purely
motor (Gallese 2002)(labelled as Motor Neurons in the figure) 10
Figure
2.8 The macaque parieto-frontal projections from mesial parietal cortex, medial
bank of the intraparietal sulcus and the surface of the superior parietal
lobule (adapted from Rizzolatti et al. 1998). Note that the Brodmann’s area 7m
corresponds to Pandya and Seltzer's (1982) area PGm... 11
Figure
2.9 The intraparietal sulcus opened to show the anatomical location of AIP in
the macaque (adapted from Geyer et al. 2000) 13
Figure
2.10 An AIP visual-dominant neuron activity under three task conditions: Object
manipulation in the light, object manipulation in the dark and object fixation
in the light. The neuron is active during fixation and holding phase when the
action is performed in light condition. However, during grasping in dark the
neuron shows no activity. The fixation of the object alone without grasping
also produces a discharge (adapted from Sakata et al. 1997a) 14
Figure
2.11 Activity the same neuron in Figure
2.10 during fixation of different objects. The neuron show selectivity
for horizontal plate (adapted from
Sakata et al. 1997a) 14
Figure
2.12 An AIP visual-dominant neuron’s axis orientation tuning and object
fixation response is shown. The neuron fires maximally during the fixation of a
vertical bar or a cylinder. The tuning is demonstrated in the lower half of the
figure (adapted from Sakata et al. 1999) 15
Figure
2.13 Response of an axis-orientation-selective (AOS) neuron in the caudal part
of the lateral bank of the intraparietal sulcus (c-IPS) to a luminous bar
tilted 45° forward (left) or 45 backward (right) in the sagittal plane. The
monkey views the bar with
binocular vision. The line segment under the histograms mark the fixation
start and the period of 1 second.
(adapted from Sakata et al. 1999) 16
Figure
2.14 The response of the same neuron in Figure 2.13, for monocular vision
conditions for the left and right eyes. (adapted from Sakata et al. 1999) 16
Figure 2.15 Orientation
tuning of a surface-orientation selective (SOS) neuron. First row: Stimuli
presented. Middle row: responses of the cell with binocular view. Last row:
responses of the cell with monocular view (adapted from Sakata et al. 1997a) 17
Figure
2.16 The reconstructed connectivity of area 7a. The thickness of the arrows
represent the strength of the connection. (adapted from Bota 2001) 20
Figure
2.17 The reconstructed connectivity of area 7b. The thickness of the arrows
represent the strength of the connection. (adapted from Bota 2001) 21
Figure
2.18 The reconstructed connectivity of area AIP. The thickness of the arrows
represent the strength of the connection. (adapted from Bota 2001) 22
Figure
3.1 Lateral view of the monkey cerebral cortex (IPS, STS and lunate sulcus
opened). The visuomotor stream for hand action is indicated by arrows (adapted
from Sakata et al., 1997) 27
Figure
3.2 AIP extracts the affordances and
F5 selects the appropriate grasp from the AIP ‘menu’. Various biases are sent
to F5 by Prefrontal Cortex (PFC) which relies on the recognition of the object
by Inferotemporal Cortex (IT). The dorsal stream through AIP to F5 is
replicated in the MNS model 28
Figure
3.3 Each of the 3 grasp types here is defined by specifying two "virtual
fingers", VF1 and VF2, which are groups of fingers or a part of the hand
such as the palm which are brought to bear on either side of an object to grasp
it. The specification of the virtual fingers includes specification of the
region on each virtual finger to be brought in contact with the object. A
successful grasp involves the alignment of two "opposition axes": the
opposition axis in the hand joining
the virtual finger regions to be opposed to each other, and the opposition axis in the object joining
the regions where the virtual fingers contact the object. (Iberall and Arbib
1990) 29
Figure
3.4 The components of hand state F(t) = (d(t),
v(t), a(t), o1(t), o2(t), o3(t), o4(t)).
Note that some of the components are purely hand configuration parameters
(namely v,o3,o4,a) whereas others are
parameters relating hand to the object. 31
Figure
3.5 The MNS (Mirror Neuron System)
model. (i) Top diagonal: a portion of the FARS model. Object features are
processed by cIPS and AIP to extract grasp affordances, these are sent on to
the canonical neurons of F5 that choose a particular grasp. (ii) Bottom right.
Recognizing the location of the object provides parameters to the motor
programming area F4 which computes the reach. The information about the reach
and the grasp is taken by the motor cortex M1 to control the hand and the arm.
(iii) New elements of the MNS model: Bottom left are two schemas, one to
recognize the shape of the hand, and the other to recognize how that hand is
moving. (iv) Just to the right of these is the schema for hand-object spatial
relation analysis. It takes information about object features, the motion of
the hand and the location of the object to infer the relation between hand and
object. (v) The center two regions marked by the gray rectangle form the core
mirror circuit. This complex associates the visually derived input (hand state)
with the motor program input from region F5canonical neurons during the
learning process for the mirror neurons. The grand schemas introduced in
section 3.2 are illustrated as the following. The “Core Mirror Circuit” schema
is marked by the center grey box; The “Visual Analysis of Hand State” schema is
outlined by solid lines just below it, and the “Reach and Grasp” schema is
outlined by dashed lines. (Solid arrows: established connections; dashed
arrows: postulated connections) 32
Figure
3.6 (a) For purposes of simulation, we
aggregate the schemas of the MNS (Mirror Neuron System) model of Figure
3.5 into three "grand schemas" for Visual Analysis of Hand State,
Reach and Grasp, Core Mirror Circuit. (b) For detailed analysis of the Core
Mirror Circuit, we dispense with simulation of the other two grand schemas and
use other computational means to provide the three key inputs to this grand
schema. 34
Figure
3.7 (Left) The final state of arm and hand achieved by the reach/grasp
simulator in executing a power grasp on the object shown. (Right) The hand
state trajectory read off from the simulated arm and hand during the movement
whose end-state is shown at left. The hand state components are: d(t), distance
to target at time t; v(t), tangential velocity of the wrist; a(t), Index and
thumb finger tip aperture; o1(t), cosine of the angle between the object axis
and the (index finger tip – thumb tip) vector; o2(t), cosine of the angle
between the object axis and the (index finger knuckle – thumb tip) vector;
o3(t), The angle between the thumb and the palm plane; o4(t), The angle between
the thumb and the index finger. 37
Figure
3.8 Grasps generated by the simulator. (a) A precision grasp. (b) A power
grasp. (c) A side grasp.. 38
Figure
3.9 (a) Training the color expert,
based on colored images of a hand whose joints are covered with distinctively
colored patches. The trained network will be used in the subsequent phase for
segmenting image. (b) A hand image (not from the training sample) is fed to the
augmented segmentation program. The color decision during segmentation is done
by consulting to the Color Expert. Note that a smoothing step (not shown) is
performed before segmentation.. 40
Figure
3.10 Illustration of the model matching system. Left: markers located by
feature extraction schema. Middle and Right: initial and final stages of model
matching. After matching is performed a number of parameters for the Hand
configuration are extracted from the matched 3D model 41
Figure
3.11 The scaling of an incomplete input to form the full spatial representation
of the hand state As an example, only one component of the hand state, the
aperture is shown. When the 66 percent of the action is completed, the
pre-processing we apply effectively causes the network to receive the stretched
hand state (the dotted curve) as input as a re-representation of the hand state
information accessible to that time (represented by the solid curve; the dashed
curve shows the remaining, unobserved part of the hand state) 44
Figure
3.12 The solid curve shows the effective input that the network receives as the
action progresses. At each simulation cycle the scaled curves are sampled (30
samples each) to form the spatial input for the network. Towards the end of the
action the networks input gets closer to the final hand state. 45
Figure
3.13 (a) A single grasp trajectory viewed from three different angles
to clearly show its 3D pattern. The wrist trajectory during the grasp is marked
by square traces, with the distance between any two consecutive trace marks
traveled in equal time intervals. (b)
Left: The input to the network. Each component of the hand state is
labelled. (b) Right: How the
network classifies the action as a power grasp: squares: power grasp output;
triangles: precision grasp; circles: side grasp output. Note that the response
for precision and side grasp is almost zero. 47
Figure
3.14 Power and precision grasp resolution. The conventions used are as in the
previous figure. (a) The curves for power and precision cross towards the end
of the action showing the change of decision of the network. (b) The left shows
the initial configuration and the right shows the final configuration of the
hand 48
Figure 3.15: (Top) Strong precision grip mirror response for a reaching
movement with a precision pinch. (Bottom) Spatial location perturbation
experiment. The mirror response is greatly reduced when the grasp is not
directed at a target object. (Only the precision grasp related activity is
plotted. The other two outputs are negligible.) 48
Figure
3.16 Altered kinematics experiment. Left: The simulator executes the grasp with
bell-shaped velocity profile. Right: The simulator executes the same grasp with
constant velocity. Top row shows the graphical representation of the grasps and
the bottom row shows the corresponding output of the network. (Only the
precision grasp related activity is plotted. The other two outputs are
negligible.) 49
Figure
3.17 Grasp and object axes mismatch experiment. Rightmost: the change of the
object from cylinder to a plate (an object axis change of 90 degrees).
Leftmost: the output of the network before the change (the network turns on the
precision grip mirror neuron). Middle: the output of the network after the object
change. (Only the precision grasp related activity is plotted. The other two
outputs are negligible.) 50
Figure
3.18 The plots show the level of mirror responses of the explicit affordance coding
object for an observed precision pinch for four cases (tiny, small, medium, big
objects). The filled circles indicate the precision activity while the empty
squares indicate the power grasp related activity.. 52
Figure
3.19 The solid curve: the precision grasp output, for the non-explicit
affordance case, directed to a tiny object. The dashed curve: the precision
grasp output of the model to the explicit affordance case, for the same object. 52
Figure
3.20: Empty squares indicate the
precision grasp related cell activity, while the filled squares represent the
power grasp related cell activity. The grasps show the effect of changing the
object affordance, while keeping a constant hand state trajectory. In each
case, the hand-state trajectory provided to the network is appropriate to the
medium-sized object, but the affordance input to the network encodes the size
shown. In the case of the biggest object affordance, the effect is enough to
overwhelm the hand state’s precision bias. 53
Figure
3.21 The graph is drawn to show the
decision switch time versus object size. The minimum is not at the boundary,
that is, the network will detect a precision pinch quickest with a medium
object size. Note that the graph does not include a point for "Biggest
object" since there is no resolution point in this case (see the final
panel of Figure 3.19) 54
Figure
3.22 The precision grasp action used to test our visual system is depicted by
superimposed frames (not all the frames are shown) 54
Figure
3.23 The video sequence used to test the visual system is shown together with
the 3D hand matching result (over each frame). Again not all the frames are
shown.. 55
Figure
3.24 The plot shows the output of the MNS model when driven by the visual
recognition system while observing the action depicted in Figure 3.22. It must
be emphasized that the training was performed using the synthetic data from the
grasp simulator while testing is performed using the hand state extracted by
the visual system only. Dashed line: Side grasp related activity; Solid line:
Precision grasp related activity. Power grasp activity is not visible as it
coincides with the time axis. 56
Figure
4.1 The elevated circular region corresponds to the area defined by the
equation (x*x+y*y) <0.25. The environment returns +1 as the reward if the
action falls into the circular region, otherwise –1 is returned. 64
Figure
4.2 The adaptation of the firing potential of the stochastic units are shown as
a series of evolving 3D maps. (left to right and top to bottom) 65
Figure
4.3. The normalized histogram of the actions generated over 60000 samples. Note
that the actions generated captured the environment’s reward distribution (see
Figure 4.1). 66
Figure
4.4 The stochastic environment’s double peaked reward distribution (see text
for the explanation) 66
Figure
4.5 Some snapshots showing the phases of learning of the layer in the
stochastic environment where the reward distribution has two peaks (see Figure
4.4). 67
Figure
4.6. The normalized histogram of 60000 data points (actions) generated by the
trained layer in the stochastic environment depicted in Figure 4.4. 68
Figure
5.1 Infant grip configurations can be divided in two categories: power and
precision grips. Infants tend to switch from power grips to precision grips as
they grow (adapted from Butterworth et al. 1997) 73
Figure
5.2 The structure of the Infant Learning to Grasp Model. The individual layers
are trained based on somatosensory feedback. 76
Figure
5.3 Hand Position layer specifies the approach direction of the hand towards
the object. The representation is allocentric (centred on the object).
Geometrically the space around the object can be uniquely specified with the
vector (azimuth, elevation, radius). The Hand Position layer generates the
vector by a local population vector computation. The locus of the local
neighbourhood is determined by the probability distribution represented in the
firing potential of Hand Position layer neurons (see Chapter 4, for details) 77
Figure
5.4:The grasp stability we used in the simulations is illustrated for a hypothetical
precision pinch grip (note that this is a simplified, the actual hand used in
the simulations has five fingers) 79
Figure
5.5 The trained model’s Hand Position layer is shown as a 3D plot. One
dimension is summed to reduce the 4D map to a 3D map. Intuitively the map says:
‘when the object is above the shoulder and in front grasp it from the bottom’ 81
Figure
5.6: The output of the trained model’s target position layer is shown as a 3D
plot. One dimension is summed to reduce the 4D map to a 3D map. The object is
on the left side of the (right handed) arm. Intuitively, the map says ‘when the
object is on the left side grasp it from the right side of the object’ 81
Figure
5.7 The learning evolution of the distribution of the Hand Position layer is
shown as a 3D plot. Note that the 1000 neurons shown represent the probability
distribution of approach directions. Initially, the layer is not trained and
responds in a random fashion to the given input. As the learning progresses,
the neurons gain specificity for this object location. 82
Figure
5.8 ILGM planned and performed a power grasp after learning. Note the
supination (and to a lesser extent extension) of the wrist required to grasp
the object from the bottom side. 83
Figure
5.9 Two learned precision grips (left: three fingered; right four fingered) are
shown. Note that the wrist configuration for each case. ILGM learned to combine
the wrist location with the correct wrist rotations to secure the object. 83
Figure
5.10 ILGM was able to generate two fingered precision grips. However these were
less than the three or four finger grips. 84
Figure
5.11 The cube on the table simulation set up. ILGM interacts with the
object with the physical constraint that it has to avoid collision with the
table. 85
Figure
5.12 ILGM learned a ‘menu’ of precision grips with the common property that the
wrist was placed well above the object. The orientation of the hand and the
contact points on the object showed some variability. Two example precision
grips are shown in the figure. 85
Figure
5.13. ILGM acquired thumb opposing index finger precision grips. 86
Figure
5.14 The three cylinder orientations and grasp attempts by the poor vision
condition. 87
Figure
5.15 The orientation match of the hand and the cylinder is illustrated. Dashed
line with diamonds: 5 months old infants; Solid line with diamonds: 9 months
old infants; Dashed line with circles: ILGM with no affordance; Solid line with
circles: ILGM with affordance (infant data from Lockman et al. (1984)). Right
panel illustrates the object orientation used for the simulation and for the
infants in this comparison.. 88
Figure
5.16 The hand orientation and cylinder orientation difference curves for
individual trials. The columns from left to right correspond to horizontal,
diagonal and vertical orientations. The upper row flat class of error
curves, lower row non-flat class for error curves (see text for
explanation) 89
Figure
5.17 The hand orientation and cylinder orientation difference curves while ILGM
was executing four types of grasp in the full-vision condition. Left two
figures are two typical error curves for the horizontal cylinder. Note that the
two horizontal case error patterns reflect the two possible grasps: from the
bottom and from the top. The third and fourth are typical error curves for the
diagonal and vertical cylinders respectively.. 90
Figure
5.18 The grasps performed after ILGM learned the association between the wrist
rotations and the object affordance (orientation) 91
Figure
6.1: The overall MNS model. The grey background rectangle shows the focus of
this chapter. In addition to the areas shown, area F2 will be posited as being
involved in grasp planning. 94
Figure
6.2 Left: precision grasp (pad opposition); Middle: Power grasp (palm
opposition); Right: Side grasp (side opposition). Each of the 3 grasp types
here is defined by specifying two ‘virtual fingers’, VF1 and VF2, which are
groups of fingers or a part of the hand such as the palm which are brought to
bear on either side of an object to grasp it. The specification of the virtual
fingers includes specification of the region on each virtual finger to be
brought in contact with the object. A successful grasp involves the alignment
of two "opposition axes": the opposition
axis in the hand joining the virtual finger regions to be opposed to each
other, and the opposition axis in the
object joining the regions where the virtual fingers contact the object
(adapted from Iberall and Arbib 1990) 95
Figure
6.3 The two possible organization of learning to grasp circuit are shown.
According to Hypothesis I, two grasping circuits exist; the phylogenetically
older one located in area F1 (hatched background) and the newer one in the
premotor cortex (solid background). According to Hypothesis II, F1 is involved
in only executing the premotor cortex instructed movements. LGM is based on the
latter hypothesis. The details of LGM are shown in Figure 6.4. Note that we
introduced area F2 for complementing the MNS structures. The visual input to
area F2 originates from MIP (not shown) and V6a. 98
Figure
6.4 The Learning to Grasp Model. F5 is implicated in all grasp related
parameters. Dashed connections indicate the direct corticospinal projections of
premotor areas. Area F5 works with area F2 and F4 to transform visual
affordances signalled by parietal areas into a grasp plan. The grasp plan is
then, relayed to primary motor cortex (F1) and spinal cord for execution. The
tactile feedback of the action is integrated in the first somatosensory cortex
(SI), which mediates the adaptation of the parietal-premotor and inter-premotor
connections. 100
Figure
6.5 The top-left shows the Hand Position layer output summed over the radius
(approach direction is encoded in spherical coordinates) as a 3D plot. The top-centre panel shows the sample
generated from the Hand Position distribution. Bottom-left shows the Wrist
Rotation layer output summed over the heading axis as a 3D plot. The
bottom-centre panel shows the parameters picked from the Wrist Rotation layer
distribution. Note that Wrist Rotation layer distribution depends on (i.e.
represents a conditional distribution) the sample picked from the Hand Position
layer. The rightmost panel shows the executed grasp.. 104
Figure
6.6 Using the same LGM used for Figure 6.5, another grasp plan is generated
(left four panels). The resulting grasp is shown on the right. By comparing the
grasp plan shown on the left four panels with of Figure 6.5’s grasp plan we see
how the selection of a different approach direction (see the centre-top panels
of both figures) changed the Wrist Orientation distribution.. 105
Figure
6.7:Two very different grasp generation from the same LGM. Upper panel:
Grasping with maximum wrist extension with some pronation. Lower panel:
Grasping with maximum wrist supination and small wrist extension. Note that the
Wrist Layer probability map is the same since the approach direction was chosen
the same (the small dots in the right most panels). 105
Figure
6.8 The grasps performed after LGM learned the association of hand rotations
with the object orientation input (full vision condition). Note that the left
panel shows a bottom side grasp. All of the shown grasp configurations
satisfied grasp stability criterion.. 107
Figure
6.9 In the poor-vision case, the hand rotation neurons in LGM show the same
response for horizontal (left panel), diagonal (centre panel) and vertical
(right panel) object presentations because of the lack of axis orientation
input. 107
Figure
6.10 When LGM has access to axis orientation information the Hand Rotation
neurons represent different plans in response to horizontal (left panel),
diagonal (centre panel) and vertical (right panel) object presentations. 108
Figure
6.11 The small object presentation produced two peaks of activity in the Hand
Position layer corresponding to the probability distribution of approach
directions. The right panel shows the executed grasp when the data generation
was localized in the area pointed by the leftmost arrow. 109
Figure
6.12: A large cube was grasped by securing the object between the thumb and the
other fingers (right panel). The Hand Position layer activity is shown on the
left panel. The neuron with largest activity is marked with an arrow... 110
Figure
6.13 The largest object presentation and grasping. The Hand Position reflects a
single reach direction as indicated with an arrow... 110
Figure
6.14:The Hand Position layer activity is superimposed to demonstrate that the
maximum activity loci are separated for each object indicating selectivity
for object size. 111
Figure
6.15 The trained Learning to Grasp Model executed grasps to objects located at
nine different locations in the workspace. The grasp locations were not used in
the training. All of the grasps shown were
stable. 114
Figure
6.16 The same model used in generating Figure
6.15 was used to generate a different set
of grasps. Again all the grasps were stable. 114
Figure
6.17 The internal mechanisms of representing and generating multiple grasp
plans are shown. Solid arrows (except object encoding) denote learned
connections while empty arrows indicate data generation. The flow of operation
starts with the presentation of the object (the bottom centre) and follows the
arrows. At the top-centre, the data generation can yield multiple approach
directions. The two possible approach directions are shown creating two streams
(left column and right column), each of which yields different grasp execution
(bottom pictures of left and right column). 115
Figure
7.1 The MNS model repartitioned to show the focus of this chapter. The grey
background marks area of interest. 118
Figure
7.2 One alternative visual control structure for manipulation is shown within
the MNS framework. The mirror neurons generate feed-forward commands. 119
Figure
7.3 Another alternative visual control structure for manipulation is shown
within the MNS framework (compare with Figure 7.2). The mirror neurons generate
feedback commands. 120
Figure
7.4 The feedback and feed-forward control view of the F5 grasping circuit,
alternative I: F5mirror neurons learn to generate feed-forward command. The
desired state is assumed to be available and is converted to a correction motor
command by F5motor-only units using stochastic gradient descent. F5canonical
neurons gate the feed-forward and feedback pairs. 121
Figure
7.5. The feedback and feed-forward control view of the F5 grasp circuit,
alternative II: F5mirror neurons learn to compute the error. The error is then
converted to a correction motor command by F5motor-only units. F5canonical
generates the feed-forward command signal. 122
Figure
7.6 The schema level view of the feedback controller. The visual processing
encapsulates the process of extracting an error based on the vision of the hand
and the object. Lower Motor Centers encapsulates the functionality involved in
transforming the motor signal into actual commands sent to muscles. 125
Figure
7.7 The leaky integrator implementation of the feedback circuit that solves the
inverse kinematics problem for precision grasping. See text for the explanation.. 126
Figure
7.8 Three grasping tasks executed by the feedback circuit proposed shown on the
upper half of the figure. The change of arm/hand configuration during the
execution is illustrated by snapshots of the arm/hand. Each hand figure is
accompanied (lower half) by the error plot. The grasp execution is stopped
(success) when the sum of finger distances to their target was less than 2mms. 128
Figure
7.9.The Visual feedback circuit generating desired trajectories for the ‘lower
level motor centre’ (implemented as a PD controller) 129
Figure
7.10 The slow (2 seconds) (lower half) and fast (0.5 seconds) (upper half)
performance of the ‘visual feedback servo’ + ‘PD controller’ system is shown.
The right hand side graphs show the tracking error (of the wrist) versus time.
In the slow case, the object can be grasped but in the fast case, it is missed.. 130
Figure
7.11 The F5 mirror neurons viewed as the memory based feed-forward controller.
The arrows below the sheet of neurons indicate outputs while the arrows coming
above the sheet indicate inputs. 133
Figure
7.12 The arm configurations that were acquired during 6 object grasping actions
are shown.
Each of the superimposed configurations is represented by a unit in the
feed-forward layer. 134
Figure
7.13 The trajectory generation with feedback and feed-forward control is
illustrated for comparison with Figure 7.8 (feedback-only system). In the lower
panel the error graphs are plotted as error versus iteration. The error is the
sum of squared distances of the fingertips to their targets. The rightmost
object was not grasped in the training (a novel object/location). Thus the
system could not make use of the feed-forward signal, approximately after
iteration 25 and switched to feedback only mode, resulting in slower
positioning of the fingers on the target locations. 135
Figure
7.14 The feedback, feed-forward and lower level motor servo
and the dynamic arm was simulated all together. Upper half: The grasp lasted
0.5 seconds. Lower half: the grasp lasted 2 seconds. The fast movement error
reduced with a factor of 6 while the slow movement reduced with a factor of 10
in terms (compare with Figure
7.10) 136
Figure
7.15 The top row demonstrates two trajectory-planning examples for grasping
without obstacle. The bottom row demonstrates how new trajectories are formed
by introduction of an obstacle as a local inhibition on the feed-forward
controller units. 137
Figure
7.16 The feed-forward unit activations for four grasp observations shown as
unit versus time. Each graph consists of 157 neurons acquired during the
learning phase (the rows). The columns represent the time. 138
Figure
7.17 A mirror neuron recorded during a grasp observation. On the left the
raster plots; on the right the histogram. The recording data shown spans 2
seconds. In addition, the hand start to move approximately at time = 1 second
indicated by the vertical bar at the centre of the raster panel (Rizzolatti and
Gallese 2001) 139
Figure
7.18 Top row: Real mirror neuron recording during a precision grasp. Bottom
row: One of the feed-forward controller unit’s responses to vision of a grasping
action. In the left panels, each raster row corresponds to a trial (Poisson
spike generation for the model). The right panels show the histograms. The
rasters aligned according to the contact of the hand with the object. 140
Figure
7.19. The similarity of a real neuron and model unit is demonstrated. Left two
panels real mirror neuron rasters and histogram. Right two panels are the model
generated rasters and histogram. A slow increasing activity is observed in both
cases. 140
Figure
7.20 Left: a sharp mirror neuron activity, which could only be replicated with
our simulator by reducing the receptive field. Right: Similar response profile
obtained from one of the feed-forward module units. 141
Figure
7.21 The population activity of feed-forward units with smaller receptive
fields. The feed-forward unit we used to match the real mirror firing profile
in Figure 7.20 is marked with an ellipse. 141
Imagine
two friends chatting over a coffee table. At the instant when one of them wants
to get a sip of coffee, he effortlessly reaches and grasps his cup, shaping his
hand according to the properties of the cup. If it were a mug, he would reach
and grab the handle so as to counteract gravity; if it were a paper cup,
probably he would grasp the cup with his whole hand covering the surface of the
cup unless the coffee is too hot, forcing him to grab the cup from its outer
rim. When he starts reaching for the cup, his friend easily understands that he
wants to drink coffee even before his hand contacts the mug. Probably the way
he reaches and shapes his hand conveys the information that he is not aiming at
other objects on the table. He could possibly even infer that his coffee is hot
before he grabs it. The situation for the two is reciprocal; they switch roles
of being observer and actor as they sip their coffee.
When
considered individually each of them is engaged in two tasks -grasping
and observing. The former is a goal directed movement while the
latter is a perceptual task. The grasping task requires the integration
of information from a variety of sources (MacKenzie and Iberall 1994). The context and visual analysis of an object determines the
general grasp plan. Proprioceptive (during reach), tactile and kinesthetic
information (after contact) are used to guide the hand and arm to complete the
grasp. Thus, the task of grasping involves, at the least, the determination of
the following information:
1. How
to conform the hand to the object. Based on object properties such as the
shape, orientation and size the questions of ‘which side of the object the hand
should approach’; ‘which fingers should be engaged’, and ’what should the
appropriate wrist rotation be’ must be answered.
2. How
to control the limbs. According to the physical properties of the environment
and the biomechanics of the limbs, a control mechanism must engage muscles to
transport the hand and shape it to match the specifications given by (1).
The
former is the problem of grasp planning; the latter is the problem of grasp
execution, which involves dynamics, that is, the adjustment of forces that
the muscles must exert to achieve the specified plan. Many other information
sources are integrated to refine the reach and grasp plan. For example,
obstacle avoidance, speed, and accuracy requirements affect the reach component
while the force requirements to secure a heavy slippery object or to handle a
delicate object affect the grasp component.
The
perceptual task, in essence, does not involve determining movement related
parameters, as no movement has to be made. Nevertheless, the observer
recognizes the action even before it is complete. Thus, the observer has to
analyze the motion of the hand and its relevance to the target to determine
whether the hand approach and preshape would yield a grasping behavior. It is
interesting to note that there is some similarity in action recognition and
action generation in terms of the underlying computations.
In
fact, if one could compare the activity of observer’s brain while he is
engaged in grasping versus while he is observing his peer grasping, one would
see that the motor related regions of the observer’s brain was active in
both observation and execution. Thus, one could conclude that the observer’s
brain mirrored the action of his peer by establishing equivalence
between the observed action and his grasp plan.
The
mechanism I caricaturized above is the focus of this thesis. The
execution-observation matching system as introduced above does exist in
monkey. There is strong evidence that human brain is also endowed with similar
mechanism.
To
be precise, this thesis investigates the cortical mechanisms involved in:
1. Translation
of a visual description of an object into an appropriate grasp plan that is,
learning to make motor plans that yield grasps that are appropriate for the
target objects
2. Mapping
of observed grasp actions into internal motor representations
3. Developmental
processes shaping neural circuits to provide the functionality (1)
4. Developmental
processes of (2), that is learning to recognize observed grasp actions
based on self-executed grasps
The
thesis analyzes (human and monkey) behavior and monkey neurophysiology from a
developmental perspective, and constantly asks the questions: what is the
underlying factor that give rise to such mechanisms? How does it shape the
basic schemas of newborns into a functional form? The aim of the thesis is to
give answers to these questions via computational models that learn and adapt starting
from minimal bootstrapping behaviors. The models and the hypotheses developed
in the thesis are based on:
1. Human
infant motor development studies
2. Human
behavioral and neuroimaging studies
3. Monkey
neurophysiology and neuroanatomy studies
The
thesis also presents significant predictions that can be experimentally tested
with the hope that experimentalists will be stimulated to conduct the
experiments suggested or design new experiments to test the model predictions
and further uncover details of the cortical mechanisms of action recognition
(mirror neurons), visuomotor learning and motor planning. The results of these
experiments then would feedback into the modeling presented here, leading to
validation (or rejection) and refinements of the models developed.
The
organization of the thesis is as follows:
Chapter
II presents the basic biological background with an emphasis on the brain
areas that will be the focus of modeling. ‘Mirror Neurons’ (Dipellegrino et al. 1992; Gallese et al.
1996; Rizzolatti et al. 1996a) of the monkey premotor cortex are introduced in this chapter.
The research on locating mirror neurons in human is also reviewed in Chapter
II.
Chapter
III develops the Mirror Neuron System (MNS) model based on the
hypothesis that self-observation of grasping movements mediates the adaptation
of parietal-premotor and premotor-premotor connections. Using simulation
results, the chapter presents predictions on the timing of mirror neuron
responses (and others) and suggests neurophysiological experiments for testing
the predictions. In addition, Chapter III introduces the grand schemas of Visual
Analysis and Reach and Grasp. The simulated 3D arm/hand of the Reach
and Grasp schema is used in other chapters to graphically display the
learned grasp actions.
Chapter
IV develops a reinforcement learning based neural architecture that is
capable of representing multiple values of a variable in terms of its
probability distribution. The probability distributions are shaped through
interaction with the environment so as to reflect the reward distribution in
the environment. Chapter IV shows how layers can be connected to build
multilayer reinforcement networks that are capable of representing conditional
probability distributions. The architecture present in Chapter IV is used by
Chapter V and Chapter VI.
Chapter
V develops the Infant Learning to Grasp Model (ILGM) based on infant
literature. ILGM is a schema level behavioral model that reproduces many infant
behaviors and produces testable hypotheses through simulation results. The
notable property is that ILGM starts with a very basic repertoire of action
mimicking neonates and show how a range of grasping categories can emerge via
explorative interaction with the environment.
Chapter
VI introduces the neurophysiological Learning to Grasp Model (LGM) as a
neural level instantiation of ILGM constrained by monkey neurophysiology and
neuroanatomy. LGM replicates existing premotor cortex findings such as the
object selectivity of F5 canonical neurons and yields testable predictions
about the grasp learning circuit in monkeys. This chapter also, poses serious
questions to neurophysiologists on the assessment used to relate neuron firing
to behavior. In particular, Chapter VI argues, by simulation results and
examples from literature, that behavior-neural activity correlation is
not an appropriate measure for investigating brain mechanisms of movement planning.
The chapter suggests an experimental methodology for deciphering the neural
substrates of movement planning suitable for finding the neuron populations
that encode the true movement control variables (parameters).
Chapter
VII asks the question, ‘why do the mirror neurons exist?’ The hypothesis of
the chapter (introduced in Chapter III) is that mirror neurons evolved
initially to provide visual feedback for manual manipulative actions, and later
became effective in recognizing actions of others. Chapter VII presents a
simplified model of grasping (planar arm/hand) with increasing degrees of
complexity in its control. First, a biologically realistic stochastic gradient
following visual feedback circuit that can perform precision grips is
developed. Then a memory based neural feed-forward circuit is introduced to
augment the visual feedback servo circuit. After assessing the behavioral
properties of the integrated visual servo circuit, the chapter analyzes the
activities of individual memory units in the feed-forward circuit. The
activities are converted into spike patterns using Poisson model of neural
firing for a direct comparison with mirror neuron data. The results indicate
that some of the feed-forward units’ firing patterns are very similar to mirror
neurons’, in spite the fact that the feed-forward activity is characterized by
a grasp error map. This supports the hypothesis that mirror neuron system
initially evolved to serve as a visual servo circuit for manual manipulation.
Chapter
VIII summarizes the key results and presents the predictions with high
impact potential.
Chapter
IX concludes the thesis by pointing out some open questions and
recommending future research directions.
In this chapter, we review the literature on the brain areas
involved in the mirror neuron system functioning and the grasp related
visuomotor transformation. We present major findings from neurophysiological
and neuroanatomical (connectivity) literature to form a background for modeling
chapters. The main regions of interest are intraparietal sulcus and premotor
areas. A supplementary review of the superior temporal sulcus and other
parietal areas is included. The posterior parietal cortex is involved in
sensory-motor transformations, combining various sensory inputs and computing
representations that are used by the motor system to generate movements. In
turn, premotor cortex is involved in generating motor plans based on parietal
representations of object affordances. The superior temporal sulcus performs
visual analysis of motion and form including biological motion providing visual
input to the parietal areas.
The macaque inferior premotor cortex is located ventral from
the spur of the arcuate sulcus (see Figure
2.1) and considered to be involved in reaching and
grasping movements (Rizzolatti et al. 1988). This region has been further
partitioned into two sub-regions: F5, the rostral region, located along the
arcuate and F4, the caudal part (see Figure
2.1).
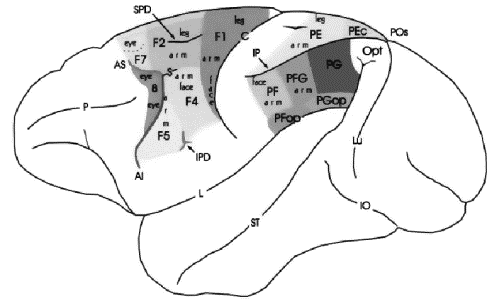
Figure 2.1 Lateral view of macaque
brain showing the areas of agranular frontal cortex and posterior parietal
cortex (adapted from Geyer et al. 2000). The
naming conventions: frontal regions, Matelli et al.(1991);
parietal regions, Pandya and Seltzer (1982)
The neurons in F4 appear to be primarily involved in the
control of proximal movements (Gentilucci et al. 1988), whereas the neurons of F5
are involved in distal control (Rizzolatti et al. 1988).
Area F5 is one of the various agranular frontal areas of
particular interest due to its complex function (Matelli 1986). In the monkey, this area
lies immediately caudal to the inferior arm of the arcuate sulcus. Stimulation
and recording experiments showed that F5 is concerned with both hand and mouth
movements. Hand movements are represented mostly in its dorsal part while mouth
movements tend to be ventrally (Rizzolatti et al. 1988). Little is known about the
functional properties of mouth neurons, however hand neurons were extensively
studied.
2.2.1.1
Motor properties
Hand neurons discharge during specific goal-related
movements such as grasping, tearing, manipulating and holding (Rizzolatti et al. 1988). Many of them are specific
for a particular type of hand movement (Rizzolatti et al. 1988). In addition, some F5 neurons
become active at the presentation of three-dimensional objects, in the absence
of any overt movement, similar to AIP neurons that become active when the
monkey fixates on a presented object. In many cases these visually triggered
discharge requires a congruence of the presented object to the grip coded by
the neuron (Murata et al. 1997a).
Rizzolatti et al. (1988) found that most F5 neuron
firings correlated with specific goal related distal motor acts rather than
with single movements made by the animal.
Using the motor acts as the classification criterion, they subdivided the
neurons into different classes such as grasping-with-the-hand-and-the-mouth,
grasping-with-the-hand and holding neurons. The discharge of many
F5 neurons depended on the way in which the hand was shaped during the motor
act. For example the three main type of neurons found by Rizzolatti et al. (1988) were precision grip, finger
prehension and whole hand prehension neurons. Furthermore, almost
all of the neurons would discharge when the action was performed with either
hand. In addition, Rizzolatti et al. (1988) reported that 20% of the
recorded neurons had visual response properties and they required
motivationally meaningful visual stimuli to be triggered. Furthermore, they
observed that, in the case of distal neurons, there was a relationship between
the type of prehension coded by the cells and the size of the stimulus
(presented object) effective in triggering the neurons. However, note that the
purely motor related neurons constitute the majority of F5 neurons (Gallese 2002).
2.2.1.2
Visual properties: canonical neurons
Murata et al. (1997a) studied the properties of
object related activity of F5 neurons. The result of their study indicates that
some F5 neurons encode object shapes in motor terms. That is, every time an
object is presented, its visual features are automatically translated into an
internal motor representation. The translation takes place whether a motor
response is required or not. Therefore, these neurons are not intention
related.
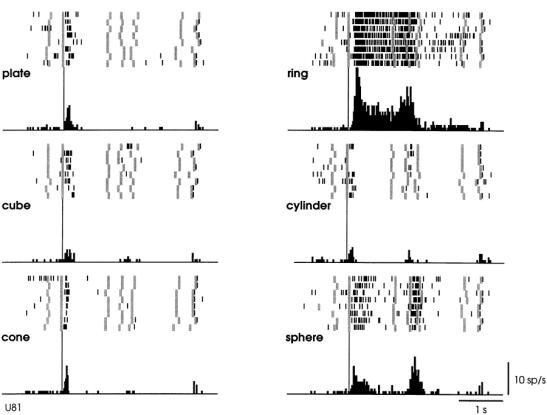
Figure 2.2 A
canonical neuron response during grasping of various objects in the dark
(left to right and top to bottom: plate, ring, cube, cylinder, cone and sphere.
The rasters and histograms are aligned with object presentation. Small grey
bars in each raster marks onset of key press, go signal, key release, onset of
object pulling, release signal, and object release, respectively. The peaks in
ring and sphere object cases correspond to the grasping of the object by the
monkey (adapted from Murata et al.
1997a)
The similarity of the AIP and F5 visual neuron responses
suggests that they may be part of a visuomotor transformation circuit. This
view is supported by the reciprocal connections between F5 and AIP (Sakata et al. 1997a). Figure 2.2 shows a canonical neuron’s response during motor
execution. To test whether the motor related activity was due to the vision of
the object, the trial was performed in the dark. The neuron was primarily
responsive for ring grasping and a lesser extend the sphere grasping. The same
neuron’s response in the object fixation, without any subsequent grasp
requirement, is shown in Figure
2.3. It is important to note that the motor preference of
the neuron is reflected in the visual fixation condition as well.
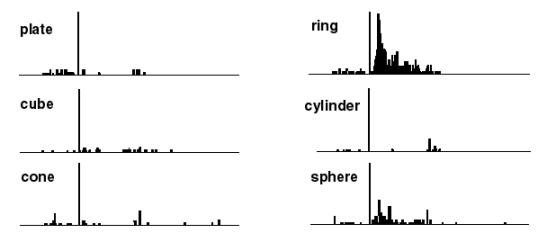
Figure 2.3 The
motor responses of the same neuron shown in Figure
2.2. The motor preference of the neuron is also carried
over to the visual preference (compare the ring and sphere histograms of both
figures) (adapted from Murata et al.
1997a)
Recording studies of the rostral part of inferior area 6
(area F5) region showed that some of the visual neurons were responsive to
action observation (Gallese et al. 1996;
Rizzolatti et al. 1996a; Dipellegrino et al. 1992). The cells with action
observation property have been located on the convexity of the bank of arcuate
sulcus.. Like other F5 neurons, mirror neurons were active when the monkey
performs a particular class of actions. However, in addition the mirror neurons
became active when the monkey observes the experimenter or another monkey
performing an action (Gallese et al. 1996;
Rizzolatti et al. 1996a; Dipellegrino et al. 1992). In most of the mirror
neurons, there was a clear relation between the coded observed and executed
action. The actions studied so far include grasping, manipulating and placing.
The congruence between the observed and executed action varied. For some of the
mirror neurons, the congruence was quite loose; for others, the general action
(e.g. grasping) and the way the action was executed (e.g. power grasp) had to
match in order to activate to neuron (Gallese et al. 1996;
Rizzolatti et al. 1996a).
An important observation was that mirror neurons required an interaction
between the experimenter and the object; the sight of the experimenter or the
object alone was not enough to trigger mirror activity. (Gallese et al. 1996;
Rizzolatti et al. 1996a)
All the neurons were studied by examining their discharge while the
experimenter performed a series of motor actions in front of the monkey. These
actions were related to food grasping and manipulation and other objects
grasping and manipulation. In order to verify whether the recorded neuron coded
specifically hand-object interactions a series of actions such as mimicking
grasping without any object, prehension actions with tools, mimicking grasp
with spatially separated object were performed. All experimenter’s actions were
repeated on different positions (e.g. left,-right, far-close). Of the 532
recorded neurons, 92 of them showed mirror property (i.e. they discharged both
when the monkey made active movements and when it observed specific meaningful
actions performed by the experimenter) (Gallese et al. 1996).
The two important aspects of the mirror neurons are (1) they
are robust, they don’t habituate and (2) the distance of the experimenter to
the monkey does not affect the response intensity of the cell. Most of the
neurons are active during observation of a single action: for example in the
study of Gallese et al. (1996). 51/92 of the cells preferred
only single action; 38/92 of the cells preferred two or three actions; 3/92 of
the cells were active for both hand or mouth grasps. The motor properties of
these neurons were indistinguishable from those of other F5 neurons. They had
preference for certain actions: 60/92 cells responded when the animal performed
only a grasping action. 9/92 cells fired when the animal grasped with his
mouth. 11/92 of cells fired for both hand and mouth grasps (Gallese et al. 1996). The remaining 14 neurons had
the distribution: tearing (2), bringing to the mouth (2), manipulating (8). The
light and dark conditions were employed for 14 cells to test whether the motor
property was a result of self-hand vision. All the tested neurons confirmed
that, the discharge was not due to self-vision (Gallese et al. 1996).
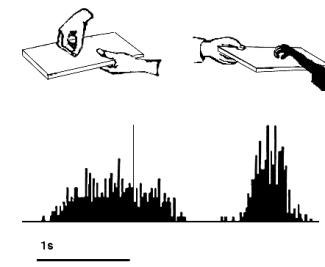
Figure 2.4 Activity of a cell during
action observation (left) and action execution (right). There is no activity in
presentation of the object during both initial presentation and bringing the
tray towards the monkey. The vertical line over the histogram indicates the
hand-object contact onset. (from Gallese et al., 1996).
Figure
2.4
shows the dual response property of mirror neurons. The recorded neuron in the
figure was silent during the presentation of the object, but started firing
when the experimenter picked up the object. The neuron, interestingly, did not
fire during the time the tray was moved towards the monkey and finally it
started firing again when the monkey picked up the object.
Note that during the period when the tray was moved towards to monkey it
could predict that he would grasp the object (Gallese et al. 1996)
Figure
2.5 shows the specificity of a grasp related mirror
neuron where the experimenter performed (A) a precision grip, (B) a whole hand
prehension, and (C) mimicked a precision grip. The notable property of this
neuron is that miming the action was not effective in activating the neuron.
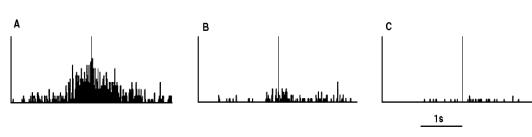
Figure 2.5 Visual response of a mirror
neuron. A. Precision grasp B. power grasp C. mimicking of precision grasp. The
vertical lines over the histograms indicate the hand-object contact onset.
(adapted from Gallese et al., 1996)
In most mirror neurons, there is a relationship between the
visual action they respond and the motor action they code. The mirror neurons
studied by Gallese et al. (1996) were divided into three,
according to their visuomotor congruence: strictly congruent, broadly
congruent and non-congruent. A neuron was labeled as strictly
congruent when the effective observed and executed actions match both in terms
of general action type (e.g. grasp) and in terms of how the action was executed
(e.g. power grasp). Figure 2.6 shows a strictly congruent neuron.

Figure 2.6 Example
of a strictly congruent manipulating mirror neuron: A) The experimenter
retrieved the food from a well in a tray. B) Same action, but performed by the
monkey. C) The monkey grasped a small piece of food using a precision grip. The
vertical lines over the histograms indicate the hand-object contact onset
(adapted from Gallese et al., 1996).
The number of strictly congruent neurons found was 29/92.
The number of broadly congruent neurons was 56/92 (Gallese et al. 1996). In the case of broadly
congruent neurons, there was a link between the executed action and the
observed preferred action. These neurons were further sub-classified according
to their motor strictness: If a broadly congruent neuron fired only for one
motor act (e.g. grasp) with only a single hand configuration (e.g. precision)
then it would be of the first type. On the other hand, if the neuron fired for
one motor act but the way the action was performed did not affect the firing
then it would be of the second type. The third and last type of broadly
congruent neurons appear to be activated by the goal of the observed action (Gallese et al. 1996). Finally, the neurons with no
apparent congruence were labeled as non-congruent (7/92). Figure 2.7 shows the classification of F5 neurons including the
mirror neuron types discussed.
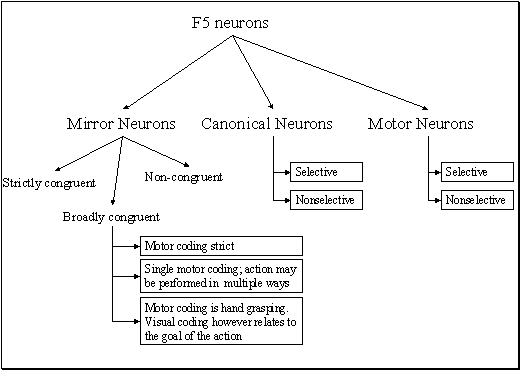
Figure 2.7 The
classification of area F5 neurons derived from published literature (Dipellegrino et al. 1992;
Gallese 2002; Gallese et al. 1996; Murata et al. 1997a; Murata et al. 1997b;
Rizzolatti et al. 1996a; Rizzolatti and Gallese 2001). All F5 neurons fire in
response to some motor action. In addition, canonical neurons fire for object
presentation while the mirror neurons fire for action observation. The majority
of hand related F5 neurons are purely motor (Gallese 2002)(labelled as Motor Neurons
in the figure)
Fogassi et al. (1998) found that area F5 was not
the only area that had mirror neurons. The rostral part of the inferior
parietal lobule of the macaque monkey (area 7b or PF) also has neurons with
similar mirror properties. Although some neurons with strict congruence of the
executed and observed action have been found, the majority of the neurons
studied had limited congruence (similarity) or no congruence at all (Fogassi et al. 1998). 8/43 PF mirror neurons were
strictly congruent; 9/43 had low level of congruence (a similarity); and the
majority (26/43) were non-congruent (Fogassi et al. 1998). The main cortical input to
area F5 comes from the inferior parietal lobe, and in particular areas AIP and
PF (Matelli 1986). The similar properties of F5
canonical neurons with AIP neurons, and F5 mirror neurons with PF neurons,
suggests that these three areas work together for visuomotor transformation and
action recognition.
Area F4 (see Figure
2.1) is connected with area F3 and to a lesser extent, to
area F6 (Geyer et al. 2000). Area F4 projects to primary
motor cortex (F1). The main parietal input to area F4 comes from VIP (Geyer et al. 2000). In area F4 the space is
coded in body-parts-centred coordinate frame (e.g. centred on the hand) (Fogassi et al. 1996). When the body-part-moves the
coordinate system follows, but when the gaze moves the coordinate frame stays
anchored on the body-part (Fogassi et al. 1996). Many of F4 neurons fire
during reaching movements of the proximal arm but not the movements of the
distal arm. The neurons usually have somatosensory receptive fields that match
the movement direction of the limb (Gentilucci et al. 1988). It is suggested that VIP-F4
circuit transforms object locations into motor plans to reach towards them as
area F4 sends descending projections to the brain stem and spinal cord (Rizzolatti et al. 1998).
Area F2 (see Figure
2.1) neurons can be grouped into three different classes:
(1) signal related neurons, (2) set-related neurons, and (3) movement-related
neurons. (see Geyer et al. 2000
for a review).
Signal related neurons are activated right after visual instruction stimuli and
have phasic response. Set-related neurons show sustained activity after the
instruction stimulus during the delay period. Movement related neurons start
firing after the trigger signal. Area F2 receives somatosensory input from
areas PEip and PEc, and visual input from areas MIP and V6A. Rizzolatti et al. (1998) suggested that F2 can use the
MIP and V6A inputs in controlling arm position during the transport of the hand
to spatial targets.
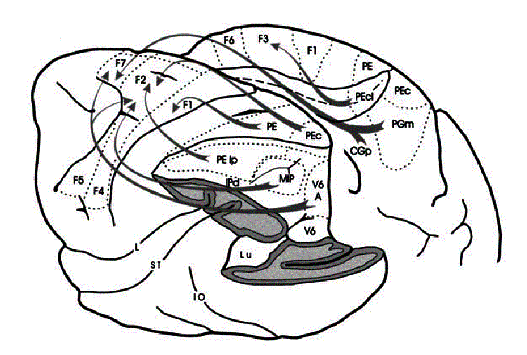
Figure 2.8 The macaque parieto-frontal
projections from mesial parietal cortex, medial bank of the intraparietal
sulcus and the surface of the superior parietal lobule (adapted from Rizzolatti et
al. 1998). Note that the Brodmann’s area 7m corresponds
to Pandya and Seltzer's (1982) area PGm
Area F7 receives inputs from area 7m (Ferraina et al. 1997a;
Ferraina et al. 1997b)
(see
Figure 2.8).
The neurons in area F7 fire in response to arm movements (Caminiti et al. 1991;
Crammond and Kalaska 1996)
or visual stimuli (Shen and Alexander
1997b).
However, in contrast to area F2, area F7 visual response does not depend on a
pending movement (di Pellegrino and Wise
1991).
It appears that the 7m-F7 circuit is important for conditional movement
selection (Geyer et al. 2000). The other projection to area
F7 is from LIP (Lewis and Van Essen
2000),
where saccade related target memory activity is represented. The neuronal
activity in LIP area can be modulated by attention and eye position (see Colby and Duhamel
1996 for a review of LIP neuron responses). Thus, LIP-F7 circuit may be
important for complex saccade control (Geyer et al. 2000).
Area F1 (see Figure
2.1) is organized somatotopically, where the body parts
that require finer movements are represented over a larger cortical surface
than the body parts that require less precision. Each neuron may contribute to
multiple spinal neuron pools. The motor parameters that are encoded by F1
neurons are usually a combination of the following physical parameters: force,
rate of change of force, joint position or the velocity of the movement (Pandya and Seltzer
1982).
However, it is possible to get meaningful physical parameters using a population
of F1 neurons. Georgopoulos et al. (Georgopoulos et al.
1982)
trained monkeys to perform radial outward reaches to a target light. Recording
over a population of primary cortex neurons they showed that each neuron fired
maximally for a direction (preferred direction), and fired less and less as the
direction deviated form the preferred direction. Given a population, the
weighted sum of the preferred direction vectors, the population vector, predicted
the monkeys reaching direction.
The subcortical input to F1 is relayed by thalamus (see Matelli et al. 1989
for the distinct nuclei projecting to F1). The corticocortical inputs
to hand area of F1 comes primarily from supplementary motor area (SMA) and to a
lesser extent from the lateral premotor cortex. The other inputs are from area
1, 2 and 5 (Ghosh et al. 1987). Approximately half of the
coriticospinal projections are formed by area F1 neurons (Dum and Strick 1991).
Area F3 is somatotopically organized where arm and leg
representations run as two oblique dorsorostral-to-ventrocaudal directions (see
Figure 2.1). In addition, area F3 has an orofacial
representation, while area F6 has only an arm representation (Luppino et al. 1991).
Areas F3 and F6 have different patterns of thalamic input
indicating that they are part of different motor loops with different functions
(Luppino et al. 1991). Cortical input to area F3
originate mainly from areas F2, F4, F5, F6 and F7, and the primary and
secondary somatosensory cortices and the posterior parietal areas PE and Peci,
and the cingulate and the primary motor cortex. On the other hand, area F6 is
mainly connected with areas F5 and F7, followed by the prefrontal and cingulate
cortex, F2, F3 and F4, and to lesser extend with the posterior areas PG, PFG
and superior temporal sulcus (Geyer et al. 2000).
In the macaques’s brain, posterior parietal cortex and the
cortex of caudal superior temporal sulcus (STS) have been subdivided into
numerous areas mainly involved in spatial analysis of the visual environment
and in the control of spatially oriented behaviour (Maioli et al. 1998). The cortex of superior
temporal sulcus (STS) contains neurons that are selective for biological motion
observation such as limb movements and full body motion. Perrett et al. (1990b) reported STS neurons that
were responsive to goal directed hand motion (Perret et al. 1990b;
Perret et al. 1990a).
PET studies showed that STS in human shows strong activation during
biologically meaningful visual stimuli (Bonda et al. 1996) including goal-directed hand
actions. In monkeys, some of the STS neurons that are triggered by biologically
meaningful stimuli have two notable properties. Firstly, these neurons show
responses to goal directed hand motion in a translation/scale/rotation
invariant way (Perret et al. 1990b;
Perret et al. 1990a).
Secondly, these neurons do not require a pictorially realistic stimulus; they respond
to point light stimuli (Perret et al. 1990b;
Perret et al. 1990a)
where the stimulus is just the movement of a small number of points. Bonda et
al. (1996) also used this kind of
stimulus -
3 lights for the arm and 2 for each finger - when they scanned the
subjects during action observation.
Based on cytoarchitectonic and connectional criteria the
inferior parietal lobule (Brodmann’s area 7) includes areas 7a, 7b and 7ip (Cavada and
Goldman-Rakic 1989).
Area 7 reaches its highest development in primates (Cavada and
Goldman-Rakic 1989).
Damage to this area can cause impairments in spatial perception, neglect of
sensory stimuli contralateral to the damage side, defects in visually guided
reaching and occulomotor control (Ratcliff 1991; Stein
1991). Cavada & Goldman-Rakic (1989) divides
area 7 in sub-areas of 7m, 7a, 7b, and 7ip. Area 7m is located on the medial
surface of the hemisphere. This corresponds to Pandya and Seltzer's (1982) area PGm. Areas 7a, 7b lie on
the convexity of the posterior parietal lobule (Cavada and Goldman-Rakic
1989).
These regions correspond to Pandya and Seltzer's (1982) PG and PF respectively .
Pandya and Seltzer's (1982) also distinguish the
subdivisions of PGop and PFop in the lateral opercular part of PG and PF and
area Opt in caudal PG. Area 7ip is situated in the posterior bank of
intraparietal sulcus and referred as POa by Pandya and Seltzer (1982). In addition, the posterior
half of 7ip corresponds to functionally defined areas VIP (Maunsell & Van
Essen, 1983) and LIP (Andersen et al.,
1985). Figure
2.9 shows the intraparietal sulcus (opened) and neighbouring parietal regions
using Pandya and Seltzer (1982) nomenclature.
The anterior part of the lateral bank of the intraparietal
sulcus (area AIP) (see Figure
2.9) is involved in extracting visual properties of
objects relevant for grasping (Sakata et al. 1997a;
Sakata et al. 1998; Sakata et al. 1995; Murata et al. 1996).
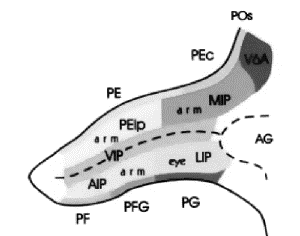
Figure 2.9 The
intraparietal sulcus opened to show the anatomical location of AIP in the
macaque (adapted from Geyer et al.
2000)
Neurons in area AIP are active either in relation to the
grasping behavior alolne or in relation to the vision of objects (Sakata et al. 1998;
Sakata et al. 1997b; Taira et al. 1990). Some of the latter type are
active exclusively for visual fixation. In one study, 21% of cells studied
responded to simply fixating an object (visual-related), others (37%) were
active only when a movement is being made to manipulate the object
(motor-related) (Taira et al. 1990). However, many cells (37%)
fell somewhere between these two extremes (visual-dominant) (Taira et al. 1990). Figure
2.10
shows the response of a visual-dominant neuron during different experimental
conditions.
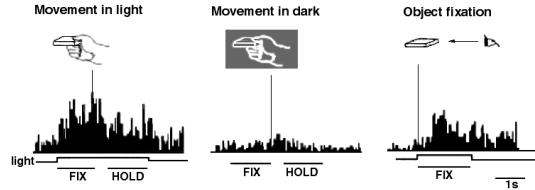
Figure 2.10 An AIP
visual-dominant neuron activity under three task conditions: Object
manipulation in the light, object manipulation in the dark and object fixation
in the light. The neuron is active during fixation and holding phase when the
action is performed in light condition. However, during grasping in dark the
neuron shows no activity. The fixation of the object alone without grasping
also produces a discharge (adapted from Sakata et al.
1997a)
The neuron shown in Figure
2.10 is active during fixation and holding phase when the
action is performed in light condition. However, in grasping-in-dark condition
the neuron shows no activity. The fixation of the object alone without grasping
also produces a discharge, however the activity is less than the
grasping-in-light condition.
.
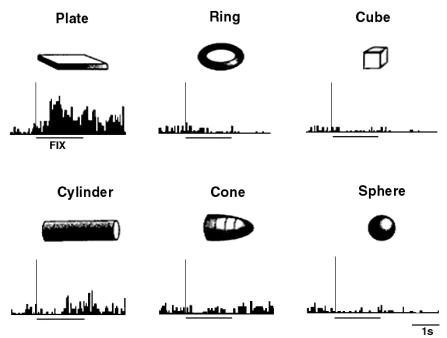
Figure 2.11 Activity
the same neuron in Figure 2.10 during fixation of different objects. The neuron show
selectivity for horizontal plate (adapted from Sakata et al. 1997a)
In addition, some of these neurons show object specificity
(object-type visual-dominant neurons) which responds to the sight of complex
objects such as a knob-in-groove and a plate-in-groove (Sakata et al. 1997a). Figure 2.11 shows response profile of the same neuron in Figure 2.10 for different objects during fixation. The neuron has
a strong preference for the plate shaped object.
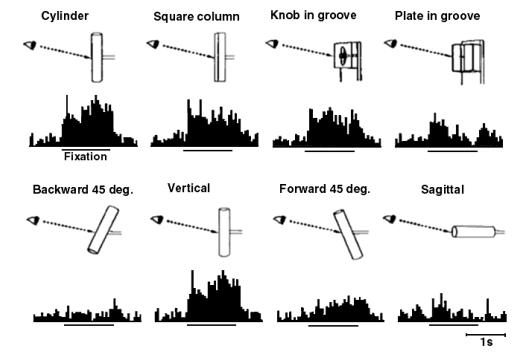
Figure 2.12 An AIP
visual-dominant neuron’s axis orientation tuning and object fixation response
is shown. The neuron fires maximally during the fixation of a vertical bar or a
cylinder. The tuning is demonstrated in the lower half of the figure (adapted from Sakata et al.
1999)
Furthermore some object-type visual-dominant neurons, show
tuning according to the orientation of the longitudinal axis or the surface
orientation of flat objects (Sakata et al. 1999;
Murata et al. 2000).
An example of an object-type visual-dominant neuron that showed tuning for
the axis orientation regardless of the shape is presented in Figure 2.12. Top row shows the strong response of the neuron to a
vertical cylinder, a square column, and a vertical knob-in-groove in the
fixation condition. The bottom row of Figure
2.12 demonstrates the tuning of the neuron for
different axis orientations.
The muscimol-induced lesions of area AIP lead to a
significant deficit in monkey's ability to grasp objects (Sakata et al. 1997a;
Gallese et al. 1994).
The grasping movements become clumsy and uncoordinated, and as a result, the
monkey is unable to shape his hand and orient his wrist appropriately for
objects that are presented. However, the monkey can still execute the basic
sequence of the task employed (Sakata et al. 1997a;
Gallese et al. 1994).
The lateral bank of
the intraparietal sulcus (c-IPS) is involved in three-dimensional analysis of
objects (Sakata et al. 1997a;
Sakata et al. 1999).
Some of these binocular visual neurons are selective for the orientation of the
axis of the objects (AOS neurons) and some are selective for the surface
orientation of the objects (SOS neurons) (Sakata et al. 1997a;
Sakata et al. 1999).
AOS neurons prefer long and thin objects as visual stimuli and are tuned to the
three-dimensional axis orientation of the objects in space. Figure
2.13
shows the response of an AOS neuron when the object is viewed in binocular
viewing condition. Figure 2.14 shows the same neuron’s response when the visual
information is limited to the left or right eye indicating that binocular cues
are important for driving the AOS neuron shown. SOS neurons prefer broad and
flat objects as visual stimuli (Sakata et al. 1999). Complementary to AOS
neurons; they are tuned to the surface orientation of objects in
three-dimensional space (see Figure 2.15).
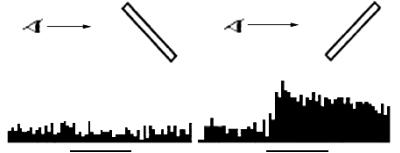
Figure 2.13
Response of an axis-orientation-selective (AOS) neuron in the caudal part of
the lateral bank of the intraparietal sulcus (c-IPS) to a luminous bar tilted
45° forward (left) or 45 backward (right) in the sagittal plane. The monkey views the bar
with binocular vision. The line segment under the histograms mark the fixation start and the
period of 1 second. (adapted from Sakata et al.
1999)
It is suggested that c-IPS is a higher center for
stereopsis, which integrates various binocular disparity signals received from
the V3 complex and other prestriate areas to represent the neural code for
geometric features of objects (Sakata et al. 1997a;
Sakata et al. 1997b).
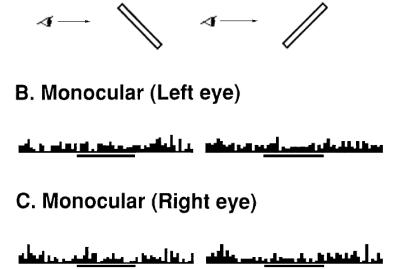
Figure 2.14 The
response of the same neuron in Figure
2.13, for monocular vision conditions for the left and
right eyes. (adapted from Sakata et al.
1999)
Sakata et al. (1997a) suggested that c-IPS could
send projections to AIP and thus, contribute to the visual adjustment of the
shape of the hand grip and/or hand orientation for manipulation and grasping. Figure 2.15 shows a SOS neuron that is selective for a surface
that is 135 degrees tilted around the sagittal axis
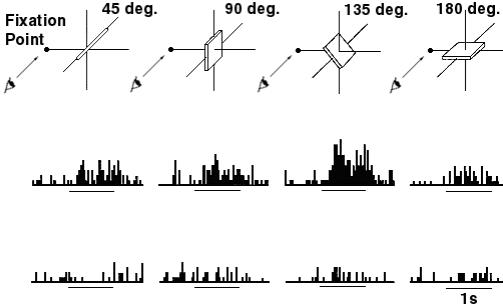
Figure 2.15 Orientation
tuning of a surface-orientation selective (SOS) neuron. First row: Stimuli
presented. Middle row: responses of the cell with binocular view. Last row:
responses of the cell with monocular view (adapted from Sakata et al.
1997a)
The intraparietal
regions VIP, MIP and LIP (see Figure 2.9) encode the space around the animal with multiple reference frames for different movement purposes (Colby and Goldberg
1999). A crude separation is that VIP is involved in ultra-near
space (less than 5cm from the face) (Colby et al. 1993b), MIP with stimuli within
reaching distance (Colby and Duhamel 1991) and LIP with far visual
stimuli (Colby and Goldberg
1999).
LIP coding has been implicated as attentional
(Gottlieb
et al. 1998),
decision-related (Shadlen
and Newsome 2001; Shadlen and Newsome 1996), visual target memory related (Gnadt and Andersen 1988) and motor intention related (Snyder et al. 2000; Snyder et al. 1997). Colby and Goldberg (1999) suggested
a unifying functional role for LIP that it encodes the representation of
salient spatial locations (with attentional tuning). They noted the
distinctive property of neurons in LIP that their firing was not
tied to any particular modality and the representation was limited
to attended objects and their locations.
Neurons in LIP have retinotopic receptive fields, where they
carry visual, memory, and saccade-related signals that describe stimuli
in terms of the distance and direction of the stimulus or saccade
location relative to the center of gaze (Colby and Goldberg
1999).
VIP neurons represent visual locations using a continuum of eye centred
to a head centred spatial reference frames (Bremmer et al. 1999; Duhamel
et al. 1997).
Eskandar and Assad (1999) found neurons with reaching-related
activity encoding stimulus features, such as location and direction
of stimulus motion. In addition, MIP neurons maintained the memory of a reach
target during the delay period of a memory-guided reach task or when
the target is obscured (Eskandar and Assad
1999; Snyder et al. 1997).
When the hand direction and the visual target direction were disassociated
through a well designed set up,
it was found that MIP neuron activity correlated more with the hand direction
than the object location. The opposite was true for LIP neurons (Eskandar and Assad
1999)
.
The experimental findings indicate that area 7a, together
with other inferior parietal lobule sectors, is involved in spatial coding.
Researchers suggested various types of spatial encoding for area 7a. Stein (1991) suggested that area 7a
represented extra-personal space. Andersen et al. (1999) suggested that area 7a
represents targets in a world-centered coordinate frame. It has been shown
that area 7a neurons are involved in the analysis of motion evoked during
locomotion or by the manipulation of
objects by the hands (Siegel and Read 1997). The different interpretation of area 7a
responses can be due to either the non-homogenous functional distributions of
neurons or due to the experimental setup differences (see the reviews: Andersen et al. 1997; Wise
et al. 1997).
It has been found that reach-related activity in area 7a
signaled specific phases of the motor performance (MacKay 1992). Further, it has been
suggested that it could be used by the frontal lobe to facilitate upcoming
elements of a motor sequence, including terminal corrections (MacKay 1992). Motter et al. (1987)
identified visually sensitive and insensitive neurons in area 7a (Motter and Mountcastle
1981; Motter et al. 1987).
The Neurons insensitive to visual stimuli comprised the fixation, oculomotor,
and projection-manipulation classes, which were suggested to be involved in
initiatives toward action (Motter and Mountcastle
1981).
Most of the visually sensitive neurons were activated from large and bilateral
response areas that excluded the foveal region. The
visually sensitive neurons were responsive to stimulus movement and direction
over a wide range of velocities. The movement vectors pointed either inward
toward the center or outward toward the perimeter of the visual field. For
bilaterally activated neurons, the vectors pointed in opposite directions in
the two half-fields (opponent vector organization). Motter and Mountcastle (1981) suggested that the neurons
could signal motion in the immediate surround.
Constantinidis and Steinmetz (1996) showed that a population of
neurons in area 7a was active during the delay period of a spatial memory task
that did not require a motor response directed toward the stimulus. Thus, it is
suggested that the activity could represent a short-term memory trace for the
spatial location of the stimuli (Constantinidis and
Steinmetz 1996).
In accordance with the spatial memory hypotheses, Maunsell
(1995) indicated that the object location coding in area 7a was capable of
representing visual stimuli without ever falling into the corresponding
receptive field.
Another functional aspect of area 7a, the attentional tuning
was studied by Constantinidis and Steinmetz (2001). Their results indicate that
area 7a neurons represent the location of the stimulus that attracts the
animal's attention and can provide the spatial information required for
directing attention to a salient stimulus in a complex scene (Constantinidis and
Steinmetz 2001).
According to our view the fundamental and unifying property
of area 7a neurons, is that they can potentially be used to monitor the
relation of body parts with respect to objects once they are fixated. A
population of neurons that detect the motion of visual stimuli inwards to (or
outwards from) the fixation point can encode the kinematics aspects (e.g.
proximity) of a movement to satisfy a goal such as reaching or grasping. There
is evidence that when humans perform reaching movements, they fixate to target
objects or obstacles to plan reach actions (Johansson et al. 2001), which can be thought of
registering the relevant locations in area 7a as a saliency map. This
proposal is supported by the fact that the removal of areas 7a, 7ab and LIP
caused marked inaccuracy in reaching in the light to visual targets but had no
effect on reaching in the dark (Rushworth et al. 1997). In contrast, the removal of
areas 5, 7b and MIP caused misreaching in the dark, but had little effect on
reaching in the light. Therefore, Rushworth et al. (1997) suggested that the two
divisions of the parietal cortex organize limb movements in distinct spatial
coordinate systems: area 7a/7ab/LIP are essential for spatial coordination of
visual motor transformations whereas areas 5/7b/MIP is essential for the
spatial coordination of arm movements in relation to proprioceptive and
efference copy information.
Other parietal areas that can be involved in hand-object
relation signals are area 7m (Ferraina et al. 1997a;
Ferraina et al. 1997b),
and area V6a and area PEc (Caminiti et al. 1999;
Battaglia-Mayer et al. 2000; Ferraina et al. 2001; Marconi et al. 2001).
Conventionally, area 7b is considered to be a somatosensory
area (Andersen et al. 1990). Robinson
and Burton (1980b) studied the somatic response
properties of neurons from area SII and area 7b. One-half of the recorded 7b
neurons responded only to somatic stimulation. Many neurons in the lateral
parts of area 7b were vigorously activated by tactile stimulation. In spite the
majority of somatic responses, some visual responses from area 7b were noted.
The visual responses of 7b neurons were not studied in detail either because it
was not the focus of interest (as in Robinson and
Burton 1980a)
or due to the complex response properties. In fact, it is possible to find
considerable unimodal visual 7b neurons as well as the neurons that respond
only to visual stimulation (Dong et al. 1994). The visual responses of 7b
neurons can be based on the signals carried by the small projections from the
visual cortical areas (Andersen et al. 1990).
Fogassi et al. (1998) studied some of area 7b
neurons’ visual properties. They found that the activity of some neurons were
triggered by the observation of various hand actions performed by the
experimenter. The neurons had motor properties similar to mirror neurons of
area F5 (see section 2.2.1.3). The congruence between the action performed by the
monkey and the observed action was usually low. The connection of area F5 with
area 7b (Fogassi et al. 1998) indicates an intimate
relation between 7b and F5 mirror neurons. Currently there are no detailed data
on 7b mirror activity. However, unpublished results (Fogassi 1999) indicate that in addition to
those neurons that have similar properties as F5 mirror neurons there exist
mirror-like neurons that fire for simple arm/hand movement observations (in
contrast to complete action observations).
According to Cavada and Goldman-Rakic (1989) 7m, 7a, 7ip are extensively
connected with a number of visual areas located on the medial surface of the
hemisphere and in the depths of parieto-occipital and intraparietal sulci.
Areas 7m, 7a, 7ip, and to a much lesser extend 7b, are reciprocally connected
with the visual temporal cortex, principally with the cortex of the superior
temporal sulcus (STS) (Cavada and Goldmanrakic
1989).
Although the density of 7b connections with the visual motion cortex of STS is
largely surpassed by the extensive connections of 7b with somatosensory areas
the interconnections of 7b with the visual regions are established through
anterior 7ip, and the transitional cortex 7ab between 7a and 7b (Cavada and Goldmanrakic
1989).
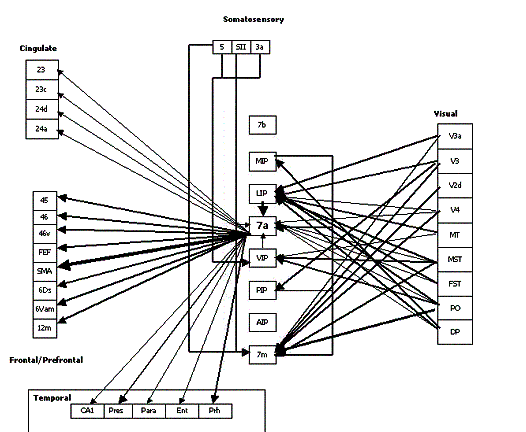
Figure 2.16 The
reconstructed connectivity of area 7a. The thickness of the arrows represent
the strength of the connection. (adapted from Bota 2001)
Findings from the same study also confirm that AIP is
connected with area 7b. Area 7ip is unique among posterior parietal areas in
its direct and indirect connections with the IT cortex (Cavada and Goldmanrakic
1989)
and may form one of the object information channel to area AIP (Sakata et al. 1997b).Figure 2.16 and Figure
2.17 shows the reconstructed connectivity of areas 7a and
7b; while Figure 2.18 shows the reconstructed connectivity of AIP (Bota 2001).
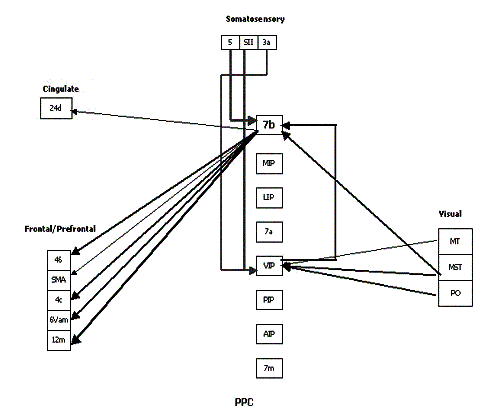
Figure 2.17 The
reconstructed connectivity of area 7b. The thickness of the arrows represent
the strength of the connection. (adapted from Bota 2001)
Andersen et al. (1990) suggests two types of
processing for area 7a, each one following a different path. First path
originates from visual area V4, which is believed to have an important role in
pattern and color processing, and reaching to area 7a. Second path is the
motion processing input originating from the middle temporal area (MT) and
relayed via medial superior temporal area (MST) or LIP (Andersen et al. 1990). MT lies on the posterior
bank of the superior temporal sulcus, while MST lies on the anterior bank of
the same sulcus (Kandel et al. 2000;
Maioli et al. 1998).
MT projects to MST and to other areas in the parietal cortex concerned with
visuospatial function. The preprocessed visual input from V1 is further elaborated
in MT, where the firing pattern of neurons reflect the speed and direction of
motion of visual targets (Kandel et al. 2000). Barnes and Pandya (1992) report that area 7a (PG-Opt)
is reciprocally connected to STS and suggest that the visuospatial analysis
that is associated with posterior intraparietal lobule could be amplified in
the multimodal regions of STS. Therefore, the neurons of multimodal areas of
the STS could be involved in analyzing the position of the body in relation to
the environment (Barnes and Pandya 1992).
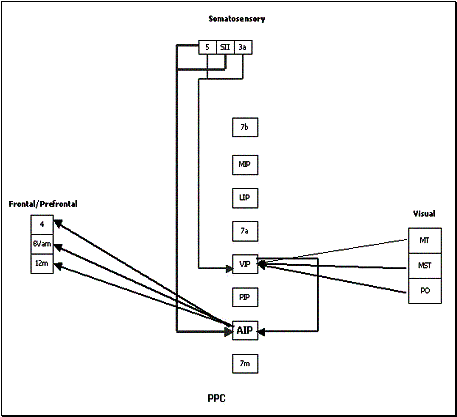
Figure 2.18 The
reconstructed connectivity of area AIP. The thickness of the arrows represent
the strength of the connection. (adapted from Bota 2001)
AIP receives input from other areas of the posterior
parietal cortex such as 7b (Neal et al. 1990). In addition, this region has
very significant recurrent cortico-cortical projections with area F5 of the
inferior premotor cortex (Matelli et al. 1994;
Sakata et al. 1997b).
Figure 3.1 illustrates the visuomotor stream for hand action as
well other related structures. Also see Figure
2.18 for the reconstructed connectivity diagram (Bota 2001) for AIP.
We conclude our discussion of anatomical connections by
summarazing the connectivity of functionally defined intraparietal regions.
Area 7a receives input from LIP (Andersen et al. 1990;
Lewis and Van Essen 2000),
MIP (Boussaoud et al. 1990;
Lewis and Van Essen 2000; Bota 2001) and VIP (reviewed in Maunsell
1995; Lewis and Van Essen 2000). Interested readers can find more details about
these connections at the NeuroHomology
Database Website
(Bota 2001 and citations
therein).
The premotor projections of these intraparietal areas include the regions F2
and F4 (Luppino et al. 1999) as reviewed by Geyer et al.(2000).
There is an unsettled debate about mirror neurons’ function.
It is suggested that mirror neurons may form the basis of understanding (Fadiga et al. 2000;
Umilta et al. 2001),
and imitation (Arbib 2001; Rizzolatti
and Arbib 1999)
and even language in human (Rizzolatti and Arbib
1998).
Thus, research for mirror neuron existence in human became necessary to support
the idea that mirror neuron involvement in. cognitive tasks.
Grafton et al. (1996) using positron emission
tomography (PET), scanned subjects under three conditions, one of them being
the control condition (object viewing). The other two were observing grasping
actions of common objects and imagining themselves doing the same grasp
actions. Grafton et al. (1996) used only precision grasps.
The imagined-minus-control and observation-minus-control results were compared.
The activation pattern was different. In their analysis, they categorized the
activations into lateral activations and medial/dorsal activations. The lateral
activation is relevant for our discussion.
In the observation condition, the activity locations were left rostral superior
temporal sulcus (STS), left inferior frontal cortex (area 45), and the left
rostral inferior parietal cortex (area 40). In addition, there was some
activation found in the rostral part of the left intraparietal sulcus. However,
the imagined grasping activated the left inferior frontal (Broca’s area or area
44) and middle frontal cortex, left caudal inferior parietal cortex (area 40).
Based on these findings, Grafton et al. (1996) suggested that the areas
active during grasping observation might form a circuit for recognition of
hand-object interactions, whereas the areas active during imagined grasping
might be a human homologue of the action observation and execution matching
system found in monkeys (mirror neurons). Their conclusion was that humans, as
in monkeys, had a similar cortical circuit that was involved in representing
observed grasping. Unfortunately, Grafton et al. (1996) did not include the
self-execution condition in the experimental setup. Therefore, it cannot be
concluded that the areas activated in this study have the dual property of the
mirror neurons (the activation during self-action and observation of the same
action performed by the demonstrator). In addition, note the discrepancy that
the human homologue of the monkey F5, namely Broca’s area (Rizzolatti and Arbib
1998),
was not activated during grasp observation but only during imagined grasping.
In another study Grafton et al. (1997) used positron emission
tomography (PET) imaging to test whether the observation of tools activates
premotor areas without any overt motor demand.
Tool observation strongly activated the left dorsal premotor cortex. Silent
tool-use naming activated Broca's area, the left dorsal premotor cortex (more
than the observation case), the left supplementary motor area and the left
ventral premotor cortex. These data indicate that, in human, F5 canonical type
of neurons may exist in the left ventral premotor cortex, which can be
triggered by object observation.
Iacoboni et al (1999)
used functional magnetic resonance imaging (fMRI) to study the brain regions
involved in imitation. Their paradigm had three observation conditions and
three observation-execution conditions. In the observation-execution
conditions, imitative and non-imitative behavior of simple finger movements was
compared. In the imitative condition, participants had to execute the observed
finger movement. In the two non-imitative conditions, participants had to
execute the same movement in response to spatial or symbolic cues The imitation
task, when contrasted to non-imitative tasks, activated three areas: the left
frontal operculum (Broca’s area or area 44), the right anterior parietal
region, and the right parietal operculum. The Broca’s area and right anterior
parietal region was also active during observation conditions. Iacoboni et al (1999)
argued that Broca's area was activated due the action-observation as Broca’s
area is the human homologue of monkey area F5 (Rizzolatti and Arbib 1998).
However, the data is not conclusive
since, the Broca’s area was active for all observation cases, not only the
action observation. Krams et al (1998) in
a similar study found that the Broca’s region was more active during action
preparation compared to action preparation-and-execution conditions. In both
conditions the visual stimuli presented was the same and consisted of a hand
drawing with a mark on a finger indicating the action to be prepared for. Krams
et al. (1998)
argued that Broca’s are was involved in action suppression (see Krams et al. 1998 for a detailed discussion). However, in both studies, the actions were intransitive; they
did involve an object to be manipulated. In contrast, the majority of mirror
neurons require an object and the action together; the miming of the action is not
effective (Gallese et al. 1996).
In one study the motor cortex was
stimulated using transcranial magnetic stimulation technique while the subjects
(1) observed an experimenter grasping 3D-objects, (2) looked at the same
3D-objects, (3) observed an experimenter tracing geometrical figures in the air
with his arm and (4) detected the dimming of a light (Fadiga et al. 1995). During the conditions of
(1)-(4) the motor evoked potentials were recorded from the hand muscles. Fadiga
et al. (1995) found that motor evoked
potentials increased when the subjects observed movements. The motor evoked
potential patterns reflected the pattern of muscle activity recorded when the
subjects executed the observed actions. Therefore Fadiga et al. (1995) concluded
that in humans there is an action observation and execution matching system,
which is similar to monkey action recognition system (mirror neurons). This
study showed that the effect of executing and observing the same action
performed by others is similar. However, the localization of action observation
and execution matching system was not possible with motor evoked potential
recordings.
Hari et al. (1998) using a different technique
(magnetoencephalogram) showed that the observation of object manipulation
activated the primary motor cortex. Hari et al. (1998) recorded neuromagnetic oscillatory activity of the human
precentral cortex while subjects were (1) idle, (2) manipulating a small object,
and (3) observing another individual performing the same task.
The left and right median nerves were stimulated alternately (inter
stimulus interval, 1.5s) at intensities exceeding motor threshold,
and the poststimulus rebound of the rolandic 15-to 25-Hz activity
was quantified (Hari et al. 1998). The rebound was diminished
during action observation as it did in action execution case (the observation
suppression magnitude was 31-46% of the suppression during object
manipulation). Hari et al. (1998) concluded that the human
primary motor cortex was activated during observation as well as
execution of the motor tasks since the 15-to 25-Hz activity mainly
originates from the precentral motor cortex.
Nishitani and Hari (2000) showed that the inferior
frontal area was active during both execution and observation of hand actions
which confirmed the existence of a mirror system in human. In contrast to
several PET studies (e.g. Grafton et al.
1996; Decety et al. 1997; Rizzolatti et al. 1996b), Broca’s area was active
during action observation while area 45 was not active. Therefore, the study of
Nishitani and Hari (2000) not only shows that the human
brain is endowed with a mirror neuron system but also supports the hypothesis
that Broca’s area is the locus of action observation and execution matching
system, which is consistent with the homology between Broca’s area and area F5 (Rizzolatti and Arbib
1998).
Buccino et al. (2001) used fMRI to localize action
recognition circuitry in humans for actions performed with different effectors.
The subjects were presented with transitive and intransitive actions performed
with mouth, hand and foot. Observation of both object- and non-object-related
actions determined a somatotopically organized activation of premotor cortex.
In addition, Buccino et al. (2001) found that during the
observation of object-related actions, an activation -also somatotopically
organized- was present in the posterior parietal lobe (Buccino et al. 2001). Buccino et al. (2001) argued
that when individuals observe object-related actions, an internal replica of
the motor act and the result of an object-related analysis are automatically
generated in the ventral premotor cortex and the parietal lobe respectively.
This result suggests that the observation and execution matching system is not
constrained to hand actions but could be a general strategy used in the primate
brain for interacting with the environment.
The posterior parietal cortex is involved in sensory-motor
transformations, combining various sensory inputs and computing representations
that are used by the motor system to generate movements. In particular AIP
extract object features relevant for grasping. Other parietal areas such as
VIP, MIP and LIP are involved in spatial aspects of object representations.
These areas project to motor and premotor cortices enabling specific movement
planning. Area F5 is involved in grasp planning while F4 is involved in
reaching movement planning. The visual areas in the superior temporal sulcus
perform visual analysis of form and motion including biological stimuli and provide parietal networks with motion
related and, for some sectors, highly processed visual input. Chapter 3 will
factor the connectivity specified in this chapter when developing Mirror Neuron
System (MNS) model. The neurophysiology of area F5 will guide the modelling presented
in this thesis throughout. We will implicate AIP and c-IPS as coding the object
affordances serving as inputs to MNS and Learning to Grasp Models (LGM) of
Chapters 5 and 6. We implicate target location schema to be represented in
areas MIP/VIP/LIP, without specifying the neural region level assignment. Area
7a will combine the hand and object related visual inputs into an internal
representation on which area 7b and F5 can be adapted to form mirror neurons.
Mirror neurons within a monkey's premotor
area F5 fire not only when the monkey performs a certain class of actions but
also when the monkey observes another monkey (or the experimenter) perform a
similar action. It has thus been argued that these neurons are crucial for
understanding of actions by others. This chapter offers the ‘hand-state’
hypothesis as a new explanation of the evolution of this capability: the basic
functionality of the F5 mirror system is to elaborate the appropriate feedback
– what we call the hand state – for
opposition-space based control of manual grasping of an object. Given this
functionality, the social role of the F5 mirror system in understanding the
actions of others may be seen as an exaptation gained by generalizing from self-hand
to other's-hand. In other words, mirror neurons first evolved to augment the
‘canonical’ F5 neurons by providing visual feedback on ‘hand state’, relating
the shape of the hand to the shape of the object.
First, we introduce the MNS (Mirror Neuron System)
model of F5 and related brain regions in terms of basic schemas. Then we
aggregate them into three ‘grand schemas’ -
Visual Analysis of Hand State, Reach and Grasp, and the Core Mirror Circuit - for
each of which we present a useful implementation. The MNS model shows how the
mirror system can learn to recognize
actions already in the repertoire of the F5 canonical neurons. The chapter, in
particular, shows how the connectivity pattern of mirror neuron circuitry can
be established through training, and that the resultant network can exhibit a
range of novel, physiologically interesting, behaviors during the process of
action recognition.
3.1
The mirror neuron system for grasping and FARS model
The macaque inferior premotor cortex has been identified as
being involved in reaching and grasping movements (Rizzolatti et al. 1988). This region has been further
partitioned into two sub-regions: F5, the rostral region, located along the
arcuate and F4, the caudal part (see Figure
3.1). The neurons in F4 appear to be primarily involved
in the control of proximal movements (Gentilucci et al. 1988), whereas the neurons of F5
are involved in distal control (Rizzolatti et al. 1988). Rizzolatti et al. (1996a;
Gallese et al. 1996). discovered a subset of F5 hand cells, which they called mirror neurons (Gallese et al. 1996;
Rizzolatti et al. 1996a).
Like other F5 neurons, mirror neurons are active when the monkey performs a
particular class of actions, such as grasping, manipulating and placing. However,
in addition, the mirror neurons become active when the monkey observes the
experimenter or another monkey performing an action. The term F5 canonical neurons is used to distinguish
the F5 hand cells which do not posses
the mirror property but are instead responsive to visual input concerning a
suitably graspable object. The canonical neurons are indistinguishable from the
mirror neurons with respect to their firing during self-action. However they
are different in their visual properties – they respond to object presentation
not action observation per se (Murata et al. 1997a).
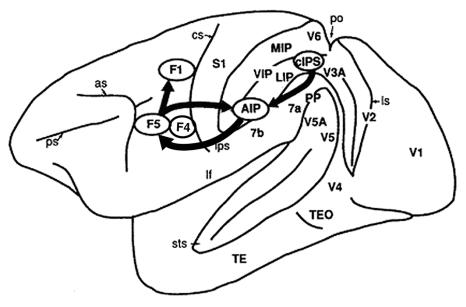
Figure 3.1 Lateral
view of the monkey cerebral cortex (IPS, STS and lunate sulcus opened). The
visuomotor stream for hand action is indicated by arrows (adapted from Sakata
et al., 1997)
Most mirror neurons exhibit a clear relation between the
observed and executed actions for which they are active. The congruence between
the observed and executed action varies. For some of the mirror neurons, the
congruence is quite loose; for others, not only must the general action (e.g.,
grasping) match but also the way the action is executed (e.g., power grasp)
must match as well. To be triggered, the mirror neurons require an interaction
between the hand motion and the object. The vision of the hand motion or the
object alone does not trigger mirror activity (Gallese et al. 1996).
It has thus been argued that the importance of mirror
neurons is that they provide a neural representation that is common to
execution and observation of grasping actions and thus that these neurons are
crucial to the social interactions of monkeys, providing the basis for understanding of actions by others
through their linkage of action and perception (Rizzolatti and Fadiga
1998).
Below, we offer the Hand-State Hypothesis, suggesting that this important role
is an exaptation of a more primitive role, namely that of providing feedback
for visually-guided grasping movements. By exaptation
we mean the exploitation of an adaptation of a system to serve a different
purpose (in this case for social understanding) than it initially developed for
(in this case, visual control of grasping). We will then develop the MNS
(Mirror Neuron System) model and show that the system can exploit its ability
to relate self-hand movements to objects to recognize the manual actions being
performed by others, thus yielding the mirror property. We also conduct a
number of simulation experiments with the model and show that these yield novel
predictions, suggesting new neurophysiological experiments to further probe the
monkey mirror system. However, before introducing the Hand-State Hypothesis and
the MNS model, we first outline the FARS model of the circuitry that includes
the F5 canonical neurons and provides the conceptual basis for the MNS model.
Studies of the anterior intraparietal sulcus (AIP; Figure 3.1) revealed cells that were activated by the sight of
objects for manipulation . In addition, this region has
very significant recurrent cortico-cortical projections with area F5 (Matelli 1984; Sakata et
al. 1997a).
In their computational model for primate control of grasping (the FARS –
Fagg-Arbib-Rizzolatti-Sakata – model), Fagg and Arbib (1998) analyzed these findings of
Sakata and Rizzolatti to show how F5 and AIP may act as part of a visuo-motor
transformation circuit, which carries the brain from sight of an object to the
execution of a particular grasp. In FARS model, the findings of Sakata (on AIP)
and Rizzolatti (on F5) were interpreted as showing that AIP represents the
grasps afforded by the object while
F5 selects and drives the execution of the grasp (Fagg and Arbib 1998). The term affordance (adapted from Gibson
1966)
refers to parameters for motor interaction that are signaled by sensory cues
without invocation of high-level object recognition processes. The model also
suggests how F5 may use task information and other constraints encoded in
prefrontal cortex (PFC) to resolve the action opportunities provided by
multiple affordances. Here we emphasize the essential components of the model (Figure 3.2) that will ground the version of the MNS model presented
below. We focus on the linkage between viewing an affordance of an object and
the generation of a single grasp.
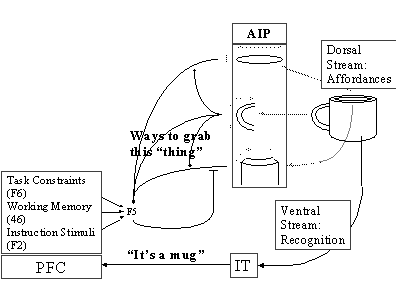
Figure 3.2 AIP extracts the
affordances and F5 selects the appropriate grasp from the AIP ‘menu’. Various
biases are sent to F5 by Prefrontal Cortex (PFC) which relies on the
recognition of the object by Inferotemporal Cortex (IT). The dorsal stream
through AIP to F5 is replicated in the MNS model
(1) The dorsal visual stream (parietal cortex) extracts
parametric information about the object being attended. It does not
"know" what the object is; it can only see the object as a set of
possible affordances. The ventral stream (from primary visual cortex to
inferotemporal cortex, IT), by contrast, recognize what the object is and
passes this information to prefrontal cortex (PFC) which can then, on the basis
of the current goals of the organism and the recognition of the nature of the
object, bias F5 to choose the affordance appropriate to the task at hand.
(2) AIP is hypothesized as playing a dual role in the
seeing/reaching/grasping process, not only computing affordances exhibited by
the object but also, as one of these affordances is selected and execution of
the grasp begins, serving as an active memory of the one selected affordance
and updating this memory to correspond to the grasp that is actually executed.
(3) F5 is hypothesized as first being responsible for
integrating task constraints with the set of grasps that are afforded by the attended
object in order to select a single grasp. After selection of a single grasp, F5
unfolds this represented grasp in time to govern the role of primary motor
cortex (F1) in its execution.
(4) In addition, the FARS model represents the way in which
F5 may accept signals from areas F6 (pre-SMA), 46 (dorsolateral prefrontal
cortex), and F2 (dorsal premotor cortex) to respond to task constraints,
working memory, and instruction stimuli, respectively, and how these in turn
may be influenced by object recognition processes in IT (see Fagg and Arbib
1988 for more details), but these aspects of the FARS model are included in MNS
model.
The key notion of the MNS model is that the brain augments
the mechanisms modeled by the FARS model, for recognizing the
grasping-affordances of an object (AIP) and transforming these into a program
of action, by mechanisms which can recognize an action in terms of the hand
state which makes explicit the relation between the unfolding trajectory of a
hand and the affordances of an object. Our radical departure from all prior
studies of the mirror system is to hypothesize that this system evolved in the
first place to provide feedback for visually-directed grasping, with the social
role of the mirror system being an exaptation as the hand state mechanisms
become applied to the hands of others as well as to the hand of the animal
itself.
As background for the Hand-State Hypothesis, we first
present a conceptual analysis of grasping. Iberall and Arbib (1990) introduced the theory of virtual fingers and opposition space. The term virtual
finger is used to describe the physical entity (one or more fingers, the
palm of the hand, etc.) that is used in applying force and thus includes
specification of the region to be brought in contact with the object (what we
might call the ‘virtual fingertip’). Figure
3.3 shows three types of opposition: those for the
precision grip, power grasp, and side opposition. Each of the grasp types is
defined by specifying two virtual fingers, VF1 and VF2, and the regions on VF1
and VF2 which are to be brought into contact with the object to grasp it. Note
that the "virtual fingertip" for VF1 in palm opposition is the
surface of the palm, while that for VF2 in side opposition is the side of the
index finger.
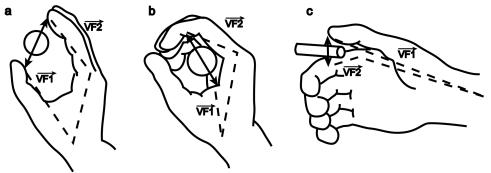
Figure 3.3 Each of
the 3 grasp types here is defined by specifying two "virtual
fingers", VF1 and VF2, which are groups of fingers or a part of the hand
such as the palm which are brought to bear on either side of an object to grasp
it. The specification of the virtual fingers includes specification of the
region on each virtual finger to be brought in contact with the object. A
successful grasp involves the alignment of two "opposition axes": the
opposition axis in the hand joining
the virtual finger regions to be opposed to each other, and the opposition axis in the object joining
the regions where the virtual fingers contact the object. (Iberall and Arbib 1990)
The grasp defines two ‘opposition axes’: the opposition axis in the hand joining the
virtual finger regions to be opposed to each other, and the opposition axis in the object joining
the regions where the virtual fingers contact the object. Visual perception
provides affordances (different ways
to grasp the object); once an affordance is selected, an appropriate opposition
axis in the object can be determined. The task of motor control is to preshape
the hand to form an opposition axis appropriate to the chosen affordance, and
to so move the arm as to transport the hand to bring the hand and object axes
into alignment. During the last stage of transport, the virtual fingers move
down the opposition axis (the ‘enclose’ phase) to grasp the object just as the
hand reaches the appropriate position.
We assert as a general principle of motor control that if a
motor plant is used for a task, then a feedback system will evolve to better
control its performance in the face of perturbations. We thus ask, as a sequel
to the work of Iberall and Arbib (1990), what information would be
needed by a feedback controller to control grasping in the manner described in
the previous section. Modeling of this feedback control is presented in Chapter
7, using a simplified hand/arm. In this chapter, our aim is to show how the
availability of such feedback signals in the primate cortex for self-action for
manual grasping can provide the action recognition capabilities which
characterize the mirror system. Specifically, we
offer the following hypothesis.
The hand-state
hypothesis: The basic functionality of the F5 mirror system is to elaborate
the appropriate feedback – what we call the hand
state – for opposition-space based control of manual grasping of an object.
Given this functionality, the social role of the F5 mirror system in
understanding the actions of others may be seen as an exaptation gained by
generalizing from self-hand to other's-hand.
The key to the MNS model, then, is the notion of hand state as encompassing data required
to determine whether the motion and preshape of a moving hand may be
extrapolated to culminate in a grasp appropriate to one of the affordances of
the observed object. Basically a mirror neuron must fire if the preshaping of
the hand conforms to the grasp type with which the neuron is associated; and
the extrapolation of hand state
yields a time at which the hand is grasping the object along an axis for which
that affordance is appropriate.
Our current representation of hand state defines a
7-dimensional trajectory
F(t) = (d(t),
v(t), a(t), o1(t), o2(t), o3(t), o4(t))
with the following components (see Figure 3.4):
Three components are hand configuration parameters:
a(t): Index finger-tip and thumb-tip aperture
o3(t), o4(t): The two angles defining
how close the thumb is to the hand as measured relative to the side of the hand
and to the inner surface of the palm
The remaining four parameters relate the hand to the object.
o1 and o2 components represent the orientation of different components of the
hand relative to the opposition axis for the chosen affordance in the object
whereas d and v represents the kinematics properties of the hand with reference
to the target location.
o1(t): The cosine of the angle between the object
axis and the (index finger tip – thumb
tip) vector
o2(t): The cosine of the angle between the object
axis and the (index finger knuckle –
thumb tip) vector
d(t): distance to target at time t
v(t): tangential velocity of the wrist
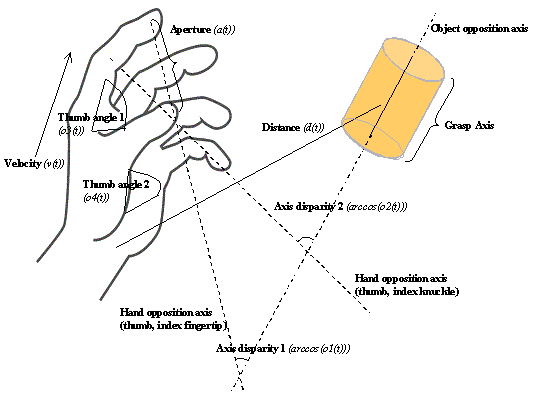
Figure 3.4 The
components of hand state F(t) = (d(t),
v(t), a(t), o1(t), o2(t), o3(t), o4(t)).
Note that some of the components are purely hand configuration parameters
(namely v,o3,o4,a) whereas others are
parameters relating hand to the object
In considering the last four variables, note that only one
or two of them will be relevant in generating a specific type of grasp, but
they all must be available to monitor a wide range of possible grasps. We have
chosen a set of variables of clear utility in monitoring the successful
progress of grasping an object, but do not claim that these and only these
variables are represented in the brain. Indeed, the brain's actual
representation will be a distributed neural code, which we predict will
correlate with such variables, but will not be decomposable into a
coordinate-by-coordinate encoding. However, we believe that the explicit
definition of hand state offered here will provide a firm foundation for the
design of new experiments in kinesiology and neurophysiology.
The crucial point is that the availability of the hand state
to provide feedback for visually-directed grasping makes action recognition
possible. Notice that we have carefully defined the hand state in terms of
relationships between hand and object (though the form of the definition must
be subject to future research). This has the benefit that it will work just as
well for measuring how the monkey’s own hand is moving to grasp an object as
for observing how well another monkey’s hand is moving to grasp the object.
This, we claim, is what allows self-observation by the monkey to train a system
that can be used for observing the actions of others and recognizing just what
those actions are.
We now present a high level view of
the MNS (Mirror Neuron System) model in terms of the set of interacting schemas (functional units: Arbib 1981; Arbib et al.
1998) shown in Figure
3.5, which define the MNS (Mirror Neuron System) model of
F5 and related brain regions. The connectivity shown in Figure 3.5 is constrained by the existing neurophysiology and
neuroanatomy of the monkey brain (reviewed in Chapter 2). We have already
introduced areas AIP and area F5, dividing the F5 grasp-related neurons into
(i) F5 mirror neurons which are, when
fully developed, active during certain self-movements of grasping by the monkey
and during the observation of a similar grasp executed by others, and (ii) F5 canonical neurons, namely those active
during self-movement and object vision but not for recognition of the action of
others. Other brain regions also play an important role in mirror neuron system
functioning in the macaque’s brain for which the readers are referred to
Chapter 2.
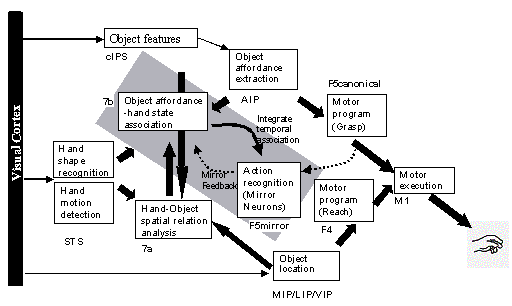
Figure 3.5 The MNS (Mirror Neuron System) model. (i) Top diagonal: a portion of
the FARS model. Object features are processed by cIPS and AIP to extract grasp
affordances, these are sent on to the canonical neurons of F5 that choose a
particular grasp. (ii) Bottom right. Recognizing the location of the object
provides parameters to the motor programming area F4 which computes the reach.
The information about the reach and the grasp is taken by the motor cortex M1
to control the hand and the arm. (iii) New elements of the MNS model: Bottom
left are two schemas, one to recognize the shape of the hand, and the other to
recognize how that hand is moving. (iv) Just to the right of these is the
schema for hand-object spatial relation analysis. It takes information about
object features, the motion of the hand and the location of the object to infer
the relation between hand and object. (v) The center two regions marked by the
gray rectangle form the core mirror circuit. This complex associates the
visually derived input (hand state) with the motor program input from region
F5canonical neurons during the learning process for the mirror neurons. The
grand schemas introduced in section 3.2 are illustrated as the following. The
“Core Mirror Circuit” schema is marked by the center grey box; The “Visual
Analysis of Hand State” schema is outlined by solid lines just below it, and
the “Reach and Grasp” schema is outlined by dashed lines. (Solid arrows:
established connections; dashed arrows: postulated connections)
The subsystem of
the MNS model responsible for the visuo-motor transformation of objects into
affordances and grasp configurations, linking AIP and F5 canonical neurons,
corresponds to a key subsystem of the FARS model reviewed above. Our task is to
complement the visual pathway via AIP by pathways directed toward F5 mirror
neurons which allow the monkey to observe arm-hand trajectories and match them
to the affordances and location of a potential target object. We will then show
how the mirror system may learn to
recognize actions already in the repertoire of the F5 canonical neurons. In
short, we will provide a mechanism whereby the actions of others are ‘recognized’
based on the circuitry involved in performing such actions. The Methods section
provides the details of the implemented schemas and the Results section
confronts the overall model with virtual experiments and produces testable
predictions.
In general, the
visual input to the monkey represents a complex scene. However, we here
sidestep much of this complexity (including attentional mechanisms) by assuming
that the brain extracts two salient sub-scenes, a stationary object and in some
cases a (possibly) moving hand. The overall system operates in two modes:
(i) Prehension: In this mode, the view
of the stationary object is analyzed to extract affordances; then under
prefrontal influence F5 may choose one of these to act upon, commanding the
motor apparatus to perform the appropriate reach and grasp based on parameters
supplied by the parietal cortex. The FARS model captures the linkage of F5 and
AIP with PFC, prefrontal cortex (Figure
3.2). In the MNS model, we incorporate the F5 and AIP
components from FARS (top diagonal of schemas in Figure 3.5), but omit IT and PFC from the present analysis.
(ii) Action recognition: In this mode,
the view of the stationary object is again analyzed to extract affordances, but
now the initial trajectory and preshape of an observed moving hand must be
extrapolated to determine whether the current motion of the hand can be
expected to culminate in a grasp of the object appropriate to one of its
affordances.
We do not
prespecify all the details of the MNS schemas. Instead, we offer a learning
model which, given a grasp that is already in the motor repertoire of the F5
canonical neurons, can yield a set of F5 mirror neurons trained to be active
during such grasps as a result of self-observation
of the monkey's own hand grasping the target object. (How such grasps may
be acquired in the first place is a topic of current research.) Consistent with
the hand-state hypothesis, the result will be a system whose mirror neurons can
respond to similar actions observed being
performed by others. The current implementation of the MNS model exploits
learning in artificial neural nets.
The heart of the
learning model is provided by the Object
affordance-hand state association schema and the Action recognition (mirror neurons) schema. These form the core mirror (learning) circuit, marked
by the gray slanted rectangle in Figure
3.5, which mediates the development of mirror neurons via
learning. The simulation results of this article will focus on this part of the
model. Section 3.4.3.1 presents in detail the neural network structure of
the core circuit. As we note further in the Discussion section, this leaves
open many problems for further research, including the development of a basic
action repertoire by F5 canonical neurons through trial-and-error in infancy
and the expansion and refinement of this repertoire throughout life.
As shown in the caption of Figure 3.5, we encapsulate the schemas shown there into the
three ‘grand schemas’ of Figure
3.6(a). These guide our implementation of MNS. Our
earlier review of the neuroscience literature in Chapter 2 justifies our
initial hypotheses, made explicit in Figure
3.5, as to where these finer-grain schemas are realized
in the monkey brain. However, after we explain these finer-grain schemas, we
will then turn to our present simulation of the three grand schemas which is
based on overall functionality. Nonetheless, the neural structure of Core
Mirror Circuit yields interesting predictions for further neurophysiological
experimentation.
3.3.2.1
Grand schema 1: reach and grasp
Object features schema: The output of this schema provides a coarse coding of geometrical features
of the observed object. It thus provides suitable input to AIP and other regions/schemas.
Object affordance extraction schema: This schema transforms its input, the
coarse coding of geometrical features of the observed object provided by the Object features schema, into a coarse
coding for each affordance of the observed object.
Motor program (grasp) schema: We identify this schema with the
canonical F5 neurons, as in the FARS model. Input is provided by AIP's coarse coding of affordances for
the observed object. We assume that the output of the schema encodes a generic
motor program for the AIP-coded affordances. This output serves as the learning
signal to the Action-recognition
(Mirror neurons) schema and drives the hand control functions of the Motor execution schema.
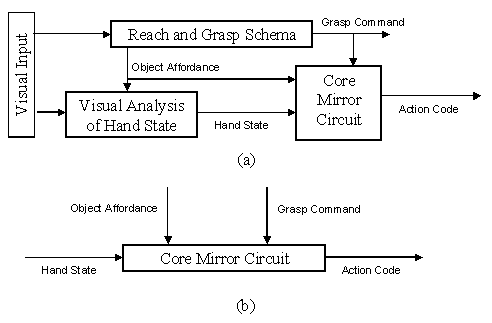
Figure
3.6 (a) For purposes of simulation, we aggregate the
schemas of the MNS (Mirror Neuron System) model of Figure 3.5 into three "grand schemas" for Visual
Analysis of Hand State, Reach and Grasp, Core Mirror Circuit. (b) For detailed
analysis of the Core Mirror Circuit, we dispense with simulation of the other
two grand schemas and use other computational means to provide the three key
inputs to this grand schema
Object location schema: The output of this schema provides, in
some body-centered coordinate frame, the location of the center of the
opposition axis for the chosen affordance of the observed object.
Motor program (reach) schema: The input is the position coded by the Object location schema, while the output
is the motor command required to transport the arm to bring the hand to the
indicated location. This drives the arm control functions of the Motor execution schema.
The motor execution schema determines the course of movements via
activity in primary motor cortex M1 and "lower" regions.
We next review the
schemas which (in addition to the previously presented Object features and Object
affordance extraction schemas) implement the visual
system of the model:
3.3.2.2
Grand Schema 2: Visual Analysis of Hand State
The hand shape recognition schema takes
as input a view of a hand, and its output is a specification of the hand shape,
which thus forms some of the components of the hand state. In the current
implementation these are a(t), o3(t) and o4(t). Note also
that we implicitly assume that the schema includes a validity check to verify
that the scene does contain a hand.
The hand motion detection schema
takes as input a sequence of views of
a hand and returns as output the wrist velocity, supplying the v(t) component
of the hand state.
The hand-object spatial relation analysis schema receives object-related signals from the
Object features schema, as well as
input from the Object Location, Hand shape recognition and Hand motion detection schemas. Its
output is a set of vectors relating the current hand preshape to a selected
affordance of the object. The schema computes such parameters as the distance
of the object to the hand, and the disparity between the opposition axes of the
object and the hand. Thus the hand state components o1(t), o2(t),
and d(t) are supplied by this schema. The Hand-Object
spatial relation analysis schema is needed because, for almost all mirror
neurons in the monkey, a hand mimicking a matching grasp would fail to elicit
the mirror neuron's activity unless the hand's trajectory were taking it toward
an object with a grasp that matches one of the affordances of the object. The
output of this visual analysis is relayed to the Object affordance-hand state association schema which drives the F5
mirror neurons whose output is a signal expressing confidence that the observed
trajectory will extrapolate to match the observed target object using the grasp
encoded by that mirror neuron.
3.3.2.3
Grand Schema 3: Core Mirror Circuit
The
action recognition schema – which is meant to correspond to the
mirror neurons of area F5 – receives two inputs in our model. One is the motor
program selected by the Motor program
schema; the other comes from the Object
affordance-hand state association schema. This schema works in two modes:
learning and recognition. When a self-executed grasp is taking place the schema
is in learning mode and the association between the observed hand-state (Object affordance-hand state association schema) and the motor program (Motor program schema) is learned. While
in recognition mode, the motor program input is not active and the schema acts
as a recognition circuit. If satisfactory learning (in terms of generalization
and the range of actions learned) has taken place via self-observation then the
schema will respond correctly while observing other’s grasp actions.
The object affordance-hand state association
schema combines all the hand related information as well as the
object information available. Thus the inputs to the schema are from Hand shape recognition (components a(t),
o3(t), o4(t)), Hand
motion detection (component v(t)), Hand-Object
spatial relation analysis (o1(t),
o2(t), d(t)) and from Object affordance extraction schemas. As
will be explained below, the schema needs a learning signal (mirror feedback).
This signal is relayed by the Action
recognition schema and, is basically, a copy of the motor program passed to
the Action recognition schema itself.
The output of this schema is a distributed representation of the object and
hand state match (in our implementation the representation is not pre-specified
but shaped by the learning process). The idea is to match the object and the
hand state as the action progresses during a specific observed reach and grasp.
In the current implementation, time is unfolded into a spatial representation
of ‘the trajectory until now’ at the input of the Object affordance-hand state association schema, and the Action
recognition schema decodes the distributed representation to form the mirror
response (again, the decoding is not pre-specified but is the result of the
back-propagation learning). In any case, the schema has two operating modes.
First is the learning mode where the schema tries to adjust its efferent and
afferent weights to ensure the right activity in the Action recognition schema. The second mode is the forward mode
where it maps the hand state and the object affordance into a distributed
representation to be used by the Action
recognition schema.
The key question
for this chapter’s modeling will be to account for how learning mechanisms may
shape the connections to mirror neuron in such a way that an action in the
motor program repertoire of the F5 canonical neurons may become recognized by
the mirror neurons when performed by others. In Chapter 5 and Chapter 6 we will
present models that can learn a repertoire of grasping actions.
To conclude this
section, we note that our modeling is subject to two quite different tests: (i)
its overall efficacy in explaining behavior and its development, which can be
tested at the level of the schemas (functional units) presented in this
article; and (ii) its further efficacy in explaining and predicting
neurophysiological data. As we shall see below, certain neurophysiological
predictions are possible given the current work, even though the present
implementation relies on relatively abstract artificial neural networks.
Having indicated the functionality and
possible neural basis for each of the schemas that will make up each grand
schema, we now turn to the implementation of these three grand schemas. We
implement the three grand schemas so that each functions correctly in terms of
its input-output relations, and so that the Core Mirror Circuit contains model
neurons whose behavior can be tested against neurophysiological data and yield
predictions for novel neurophysiological experiments. The Core Mirror Circuit
is thus the heart of MNS model that enables us to produce testable predictions
(Figure 3.6b), but in order to study it, there must be an
appropriate context, necessitating the construction of the kinematically
realistic Reach and Grasp Simulator and the Visual Analyzer for Hand State. The
latter will first be implemented as an analyzer of views of human hands, and
then will have its output replaced by simulated hand state trajectories to
reduce computational expense in our detailed analysis of the Core Mirror.
We first discuss the Reach and Grasp
Simulator that corresponds to the whole reach and grasp command system shown at
the right of the MNS diagram (Figure
3.5). The simulator lets us move from the representation
of the shape and position of a (virtual) 3D object and the initial position of
the (virtual) arm and hand to a trajectory that successfully results in
simulated grasping of the object. In other words the simulator plans a grasp
and reach trajectory and executes it in a simulated 3D world (see Chapters 5
and 6 for neural realization of this schema). Trajectory planning (for
example Kawato and Gomi 1992; Kawato et al. 1987; Jordan and Rumelhart 1992;
Karniel and Inbar 1997; Breteler et al. 2001) and control of
prehension(Hoff
and Arbib 1993; see Wolpert and Ghahramani 2000 for a review), and their adaptation,
have been widely studied. However, our simulator is not adaptive - its
sole purpose is to create kinematically realistic actions. A similar reach and
grasp system was proposed (Rosenbaum
et al. 2001; Rosenbaum et al. 1999) where a movement is
planned based on the constraint hierarchy, relying on obstacle avoidance and
candidate posture evaluation processes (Meulenbroek et al. 2001). However, the
arm and hand model was much simpler than ours as the arm was modeled as a 2D
kinematics chain. Our Reach/Grasp Simulator is a non-neural extension of FARS
model functionality to include the reach component. It controls a virtual 19
degrees DOF arm/hand (3 at the shoulder, 1 for elbow flexion/extension, 3 for
wrist rotation, 2 for each finger joints with additional 2 DOFs for thumb one
to allow the thumb to move sideways, and the other for the last joint in the
thumb) and provides routines to perform realistic grasps. This kinematics
realism is based on the literature of primate reach and grasp experiments (Jeannerod
et al. 1995; for human see Hoff and Arbib 1993 and citations therein; for
monkey see Roy et al. 2000). During a typical
reach to grasp movement, the hand will follow a ‘bell-shaped’ velocity profile
(a single peaked velocity curve). The kinematics of the aperture between
fingers used for grasping also exhibits typical characteristics. The aperture
will first reach a maximum value that is larger than the aperture required for
grasping the object and then as the hand approaches to the target the hand
encloses to match the actual required aperture for the object. It is also
important to note that in grasping tasks the temporal pattern of reaching and
grasping is similar in monkey and human (Roy
et al. 2000). Of course, there are
inter-subject and inter-trial variability in both velocity and aperture
profiles (Marteniuk
and MacKenzie 1990). Therefore in our
simulator we captured the qualitative aspects of the typical reach and grasp
actions, namely that the velocity profiles have single peaks and that the hand
aperture has a maximum value which is larger than the object size (see Figure 3.7, curves a(t) and v(t) for sample aperture and
velocity profiles generated by our simulator) . A grasp is planned by first
setting the operational space constraints (e.g., points of contact of fingers
on the object) and then finding the arm-hand configuration to fulfill the
constraints. The latter is the inverse kinematics problem. The simulator solves
the inverse kinematics problem by simulated gradient descent with noise added
to the gradient (see Appendix 11.1.2 for a grasp planning example). Once the hand-arm configuration
is determined for a grasp action, then the trajectory is generated by warping
time using a cubic spline. The parameters of the spline are fixed and
determined empirically to satisfy aperture and velocity profile requirements. Within the simulator, it is possible to adjust the
target identity, position and size manually using a GUI or automatically by the
simulator as, for example, in training set generation.
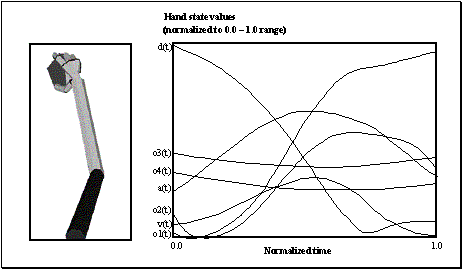
Figure
3.7 (Left)
The final state of arm and hand achieved by the reach/grasp simulator in
executing a power grasp on the object shown. (Right) The hand state trajectory
read off from the simulated arm and hand during the movement whose end-state is
shown at left. The hand state components are: d(t), distance to target at time
t; v(t), tangential velocity of the wrist; a(t), Index and thumb finger tip
aperture; o1(t), cosine of the angle between the object axis and the (index
finger tip – thumb tip) vector; o2(t), cosine of the angle between the object
axis and the (index finger knuckle – thumb tip) vector; o3(t), The angle
between the thumb and the palm plane; o4(t), The angle between the thumb and
the index finger
Figure
3.7 (left) shows the end state of a power grasp, while Figure 3.7 (Right) shows the time series for the hand state
associated with this simulated power grasp trajectory. For example, the curve
labeled d(t) show the distance from the hand to the object decreasing until the
grasp is completed; while the curve labeled a(t) show how the aperture of the
hand first increases to yield a safety margin larger than the size of the
object and then decreases until the hand contacts the object.
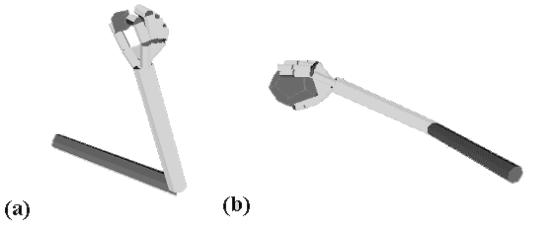

Figure
3.8 Grasps
generated by the simulator. (a) A precision grasp. (b) A power grasp. (c) A
side grasp
Figure
3.8(a) shows the virtual hand/arm holding a small cube in
a precision grip in which the index
finger (or a larger "virtual finger") opposes the thumb. The power
grasp (Figure 3.8(b)) is usually applied to big objects and
characterized by the hand’s covering the object, with the fingers as one
virtual finger opposing the palm as the other. In a side grasp (Figure 3.8(c)), the thumb opposes the side of another finger. To
clarify the type of heuristics we use to generate the grasp, Appendix 11.1.2 outlines the grasp planning and execution for a
precision pinch.
Visual Analysis of Hand State Schema is a
non-neurophysiological implementation of a visual analysis system to validate
the extraction of hand parameters from a view of a hand, by recovering the
configuration of a model of the hand being seen. The hand model is a three
dimensional 14 degrees of freedom (DOF) kinematic model, with a 3-DOF joint for
the wrist, two 1-DOF joints (metacarpophalangeal and distalinterphalangeal) for
each of the four fingers, and finally a 1-DOF joint for the metacarpophalangeal
joint, and a 2-DOF joint for the carpometacarpal joint of the thumb. Note the
distinction between ‘hand configuration’ which gives the joint angles of the
hand considered in isolation, and the ‘hand state’ which comprises 7 parameters
relevant to assessing the motion and preshaping of the hand relative to an
object. Thus, the hand configuration provides some, but not all, of the data
needed to compute the hand state.
To lighten the load of building a visual
system to recognize hand features, we marked the wrist and the articulation
points of the hand with colors. We then used this color-coding to help
recognize key portions of the hand and used this result to initiate a process
of model matching. Thus, the first step of the vision problem was color
segmentation, after which the three dimensional hand shape was recovered.
3.4.2.1
Color segmentation and feature extraction
One needs color segmentation to locate the colored regions on the image. Gray
level segmentation techniques cannot be used in a straightforward way because
of the vectorial nature of color images (Lambert
and Carron 1999). Split-and-Merge is a
well-known image segmentation technique in image processing (Sonka
et al. 1993), recursively splitting
the image into smaller pieces until some homogeneity criterion is satisfied as
a basis for reaggregation into regions. In our case, the criterion is having
similar color throughout a region. However, RGB (Red-Green-Blue) space is not
well suited for this purpose. HSV (Hue-Saturation-Value) space is better suited
since hue in segmentation processes usually corresponds to human perception and
ignores shading effects (Russ
1998 Chapters 1 and 6). However, the
segmentation system we implemented with HSV space, although better than the RGB
version, was not satisfactory for our purposes. Therefore, we designed a system
that can learn the best color space.
Figure
3.9(a) shows the training phase of the color expert system, which is a (one
hidden-layer) feed-forward network with sigmoidal activation function. The
learning algorithm is back-propagation with momentum and adaptive learning
rate. The given image is put through a smoothing filter to reduce noise in the
image before training. Then the network is given around 100 training samples
each of which is a vector of ((R, G, B), perceived
color code) values. The output color code is a vector consisting of all
zeros except for one component corresponding to the perceived color of the patch. The training builds an internal
non-linear color space from which it can unambiguously tell the perceived
color. This training is done only at the beginning of a session to learn the
colors used on the particular hand. Then the network is fixed as the hand is
viewed in a variety of poses.
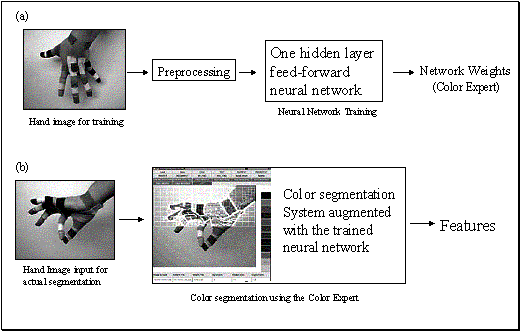
Figure
3.9 (a) Training the color
expert, based on colored images of a hand whose joints are covered with
distinctively colored patches. The trained network will be used in the
subsequent phase for segmenting image. (b) A hand image (not from the training
sample) is fed to the augmented segmentation program. The color decision during
segmentation is done by consulting to the Color Expert. Note that a smoothing
step (not shown) is performed before segmentation
Figure
3.9(b) illustrates the actual segmentation process using
the ‘color expert’ to find each region of a single (perceived) color (see
Appendix 11.1.1 for details). The output of the algorithm is then
converted into a feature vector with a corresponding confidence vector giving a
confidence level for each component in the feature vector. Each finger is marked with two patches of the same
color. Sometimes it may not be possible to determine which patch corresponds to
the fingertip and which to the knuckle. In those cases, the confidence value is
set to 0.5. If a color is not found (e.g., the patch may be obscured), a zero
value is given for the confidence. If a unique color is found without any
ambiguity then the confidence value is set to 1. The segmented centers of
regions (color markers) are taken as the approximate articulation point
positions. To convert the absolute color centers into a feature vector we
simply subtract the wrist position from all the centers found and put the
resulting relative (x,y) coordinate into the feature vector (but the wrist is
excluded from the feature vector as the positions are specified with respect to
the wrist position).
3.4.2.2
3D hand model matching
Our model matching algorithm uses the
feature vector generated by the segmentation system to attain a hand
configuration and pose that would result in a feature vector as close as
possible to the input feature vector (Figure
3.10). The scheme we use is a simplified version of Lowe’s
(1991); see Holden (1997) for a review of other
hand recognition studies.
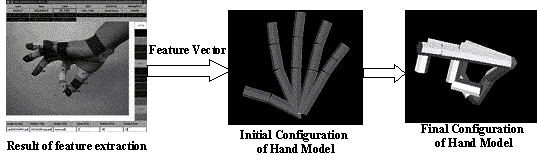
Figure
3.10
Illustration of the model matching system. Left: markers located by feature
extraction schema. Middle and Right: initial and final stages of model
matching. After matching is performed a number of parameters for the Hand
configuration are extracted from the matched 3D model
The matching algorithm is based on
minimization of the distance between the input feature and model feature
vector, where the distance is a function of the two vectors and the confidence
vector generated by segmentation system. Distance minimization is realized by
hill climbing in feature space. The method can handle occlusions by starting
with ‘don't cares’ for any joints whose markers cannot be clearly distinguished
in the current view of the hand
The distance between two feature vectors F and G is computed as follows:
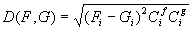
where subscripting denotes components and Cf, Cg denotes the
confidence vectors associated with F
and G. Given this result of the
visual processing – our hand shape
recognition schema – we can clearly read off the following components of
the hand state, F(t):
a(t): aperture of the virtual fingers
involved in grasping
o3(t), o4(t): the two
angles defining how close the thumb is to the hand as measured relative to the
side of the hand and to the inner surface of the palm (see Figure 3.4). The remaining components can easily be computed
once the object affordance and location is known. The computation of the
components:
d(t): distance to target at time t, and
v(t): tangential velocity of the wrist
o1(t): Angle between the object
axis and the (index finger tip – thumb
tip) vector
o2(t): Angle between the object axis
and the (index finger knuckle – thumb tip)
vector
constitute
the tasks of the hand-object spatial
relation analysis schema and the hand
motion detection schema. These require visual inspection of the relation
between hand and target, and visual detection of wrist motion, respectively. Section
3.5.3 presents justifies the visual analysis of hand state
schema by showing MNS performance when the hand state was extracted by the
described visual recognition system based on a real video sequence. However, when we turn to modeling the Core Mirror
Circuit in the next section, we will
not use this implementation of visual analysis of hand state but instead, to
simplify computation, we will use synthetic output generated by the reach/grasp
simulator to emulate the values that could be extracted with this visual
system. Specifically, we use the hand/grasp simulator to produce both (i) the
visual appearance of such a movement for our inspection (Figure
3.7, left), and (ii) the hand state trajectory associated
with the movement (Figure 3.7, right). Especially, for training we need to generate
and process too many grasp actions, which makes it impractical to use the
visual processing system without special hardware as the computational time
requirement is too high. Nevertheless, we need
to show the similarity of the data from the visual system and the simulator: We
have already shown that the grasp simulator generates aperture and velocity
profiles that are similar to those in real grasps. Of course, there is still
the question of how well the our visual system can extract these features and
more importantly how similar are the other components of the hand state that we
did not specifically craft to match the real data. Positive evidence will be
presented in Section 3.5.3.
As diagrammed in Figure 3.6(b), our detailed analysis of the core mirror
circuit does not require simulation of the visual analysis of hand state and of
reach and grasp so long as we ensure that it receives the appropriate inputs.
Thus, we supply the object affordance and grasp command directly to the network
at each trial. (Actually, we conduct experiments to compare performance with
and without an explicit input which codes object affordance.) For the hand
state input, rather than providing visual input to the visual analysis of hand
state schema and have it compute the hand state input to the core mirror
circuit, we use our reach and grasp simulator to simulate the performance of
the observed primate – and from this
simulation we extract (as in Figure 3.7) both a graphical display of the arm and hand movement that
would be seen by the observing
monkey, as well as the hand state trajectory that would be generated in its
brain. We thus use the time-varying hand state trajectory generated in this way
to provide the input to the model of the core mirror circuit of the observing
monkey without having to simultaneously model its visual analysis of hand
State. Thus, we have implemented the core mirror circuit in terms of neural
networks using as input the synthetic data on hand state that we gather from
our reach and grasp simulator (however see Section 3.5.3 for a simulation with real data extracted by our
visual system). Figure 3.13 shows an example of the recognition process together
with the type of information supplied by the simulator.
In our
implementation, we used a feed-forward neural network with one hidden layer. In
contrast to the previous sections, we can here identify the parts of the neural
network as Figure 3.5 schemas in a one-to-one fashion. The hidden layer of
the model neural network corresponds to the object
affordance-hand state association schema, while the output layer of the
network corresponds to the action
recognition schema (i.e., we identify the output neurons with the F5 mirror
neurons). In the following
formulation MR (mirror response) represents the output of the action recognition schema, MP
(motor program) denotes the target of the network (copy of the output of motor program (grasp) schema). X denotes the input
vector applied to the network, which is the transformed Hand State (and the
object affordance). The transformation applied is described in the next
subsection. The learning algorithm used is back propagation (Rumelhart et al. 1986) with momentum term. The formulation is
adapted from (Hertz et al. 1991).
Activity
propagation (forward pass)
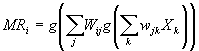
Learning weights
from input to hidden layer
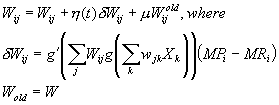
Learning weights
from hidden to output layer
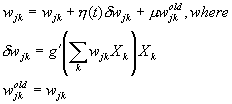
The squashing
function g we used was 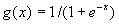 .
.  and
and 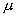 are the learning rate and the momentum coefficient
respectively. In our simulations, we adapted
are the learning rate and the momentum coefficient
respectively. In our simulations, we adapted 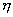 during training such that if the output error was
consistently decreasing then we increased
during training such that if the output error was
consistently decreasing then we increased 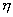 . Otherwise, we decreased
. Otherwise, we decreased 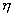 . We kept
. We kept 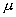 as a constant set to 0.9. W is the 3x(6+1) matrix of real numbers representing the
hidden-to–output weights. w is the 6x(210+1) (6x(220+1) in the
explicit affordance coding case) matrix of real numbers representing the input
to hidden weights, and X is the 210+1 (220+1 in explicit
affordance coding case) component input vector representing the hand state
(trajectory) information. (The extra +1 comes from the fact that the
formulation we used hides the bias term required for computing the output of a
unit in the incoming signals as a fixed input clamped to 1)
as a constant set to 0.9. W is the 3x(6+1) matrix of real numbers representing the
hidden-to–output weights. w is the 6x(210+1) (6x(220+1) in the
explicit affordance coding case) matrix of real numbers representing the input
to hidden weights, and X is the 210+1 (220+1 in explicit
affordance coding case) component input vector representing the hand state
(trajectory) information. (The extra +1 comes from the fact that the
formulation we used hides the bias term required for computing the output of a
unit in the incoming signals as a fixed input clamped to 1)
3.4.3.2
Temporal to spatial transformation
The input to the
network was formed in a way to allow encoding of temporal information without
the use of a dynamic neural network, and solved the scaling problem. The input
at any time represented the entire input from the start of the action until the
present time t. To form the input vector, each of the seven components of the
hand state trajectory to time t is fitted by a cubic spline (see Kincaid and Cheney 1991 for a
formulation), and the splines are then sampled at 30 uniformly spaced
intervals. The hand state input is then a vector with 210 components: 30
samples from the time-scaled spline fitted to the 7 components of the
hand-state time series. Note then that no matter what fraction t is of the
total time T of the entire trajectory, the input to the network at time t
comprises 30 samples of the hand-state uniformly distributed over the interval
[0, t]. Thus the sampling is less densely distributed across the
trajectory-to-date as t increases from 0 to T.
An alternative
approach would be to use an SRN (simple recurrent neural network) style
architecture to recognize hand state trajectories. However, this raises an
extra quantization or segmentation step to convert the continuous hand state
trajectories to discrete states. With our approach, we avoid this extra step
because the quantization is implicitly handled by the learning process.
Figure 3.11 The scaling of an incomplete input to form the full spatial
representation of the hand state As an example, only one component of the hand
state, the aperture is shown. When the 66 percent of the action is completed,
the pre-processing we apply effectively causes the network to receive the
stretched hand state (the dotted curve) as input as a re-representation of the
hand state information accessible to that time (represented by the solid curve;
the dashed curve shows the remaining, unobserved part of the hand state)
Figure 3.11 demonstrates the preprocessing we use to transform
time varying hand state components into spatial code. In the figure, only a
single component (the aperture) is shown as an example. The curve drawn by the
solid line indicates the available information when the 66% of the grasp action
is completed. In reality a digital computer (and thus the simulator) runs in
discrete time steps, so we construct the continuous curve by fitting a cubic
spline to the collected samples for the value represented (aperture value in
this case). Then we resample 30 points from the (solid) curve to form a vector
of size 30. In effect, this presents the network with the stretched spline
shown by the dotted curve. This method has the desirable property of avoiding
the time scaling problem to establish the equivalence of actions that last
longer than shorter ones, as it is the case for a grasp for an object far from
to the hand compared to a grasp to a closer object. By comparing the dotted
curve (what the network sees at t = 0.66) with the ‘solid + dashed’ curve (the
overall trajectory of the aperture) we can see how much the network’s input is
distorted. As the action gets closer to its end the discrepancy between the
curves tends to zero. Thus, our preprocessing gives rise to an approximation to
the final representation when a certain portion or more of the input is seen. Figure 3.12 samples the temporal evolution of the spatial input
the network receives.
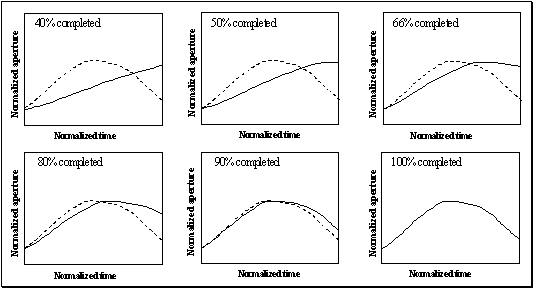
Figure 3.12 The solid curve shows the effective input that the network receives
as the action progresses. At each simulation cycle the scaled curves are
sampled (30 samples each) to form the spatial input for the network. Towards
the end of the action the networks input gets closer to the final hand state
3.4.3.3
Neural network training
The training set
was constructed by making the simulator perform various grasps in the following
way.
(1) The objects
used were a cube of changing size (a generic size cube scaled by a random scale
factor between 0.5 and 1.5), a disk (approximated as a thin prism), a ball
(approximated as a dodecahedron) again scaled randomly by a number between 0.75
and 1.5. In this particular trial, we did not change the disk size. In the
training set formation, a certain object always received a certain grasp
(unlike the testing case).
(2) The target
locations were chosen form the surface patches of a sphere centered on the
shoulder joint. The patch is defined by bounding meridian (longitude) and
parallel (latitude) lines. The extent of the meridian and parallel lines was
from -45° to 45°. The step chosen was 15°. Thus the simulator made 7x7 = 49
grasps per object. The unsuccessful grasp attempts were discarded from the
training set. For each successful grasp, two negative examples were added to
the training set in the following way. The inputs (group of 30) for each
parameter are randomly shuffled. In this way, the network was forced to learn
the order of activity within a group rather than learning the averages of the
inputs (note that the shuffling does not change mean and variance). The second
negative pattern was used to stress that the distance to target was important.
The target location was perturbed and the grasp was repeated (to the original
target position).
Finally, our last modification
in the backpropagation training algorithm was to introduce a random input
pattern (totally random; no shuffling) on the fly during training and ask the
network to produce zero output for those patterns. This way we not only biased
the network to be as silent as possible during ambiguous input presentation but
also gave the network a higher chance to reach global minima.
It should be
emphasized that the network was trained using the complete trajectory of the
hand state (analogous to adjusting synapses after the self-grasp is completed).
During testing, in contrast, the prefixes of a trajectory were used (analogous
to predictive response of mirror neurons while observing a grasp action). The
network thus yielded a time-course of activation for the mirror neurons. As we
shall see in the Results section, initial prefixes yields little or no mirror
neuron activity, and ambiguous prefixes may yields transient activity of the
‘wrong’ mirror neurons.
We thus need to
make two points to highlight the contribution of this study:
(1) It is, of
course, trivial to train a network to pair complete trajectories with the final
grasp type. What is interesting here is that we can train the system on the
basis of final grasp but then observe the whole time course of mirror neuron
activity, yielding predictions for neurophysiological experiments by
highlighting the importance of the timing
of mirror neuron activity.
(2) It is commonly
understood that the training method used here, namely back-propagation, is not
intended to be a model of the cellular learning mechanisms employed in cerebral
cortex. This might be a matter of concern were we intending to model the time
course of learning, or analyze the effect of specific patterns of neural
activity or neuromodulation on the learning process. However, our aim here is
quite different: we want to show that the connectivity of mirror neuron
circuitry can be established through training, and that the resultant network
can exhibit a range of novel, physiologically interesting, behaviors during the
process of action recognition. Thus, the actual choice of training procedure is
purely a matter of computational convenience, and the fact that the method
chosen is non-physiological does not weaken the importance of our predictions concerning
the timing of mirror neuron activity.
In this study, we
experimented with two types of network. The first has only the hand state as
the network input. We call this version the non-explicit
affordance coding network since the hand state will often imply the object
affordance in our simple grasp world. The second network we experimented with –
the explicit affordance coding network
- has affordance coding as
one set of its inputs. The number of hidden layer units in each case was chosen
as 6 and there were 3 output units, each one corresponding to a recognized
grasp
We first present
results with the MNS model implemented without an explicit object affordance
input to the core mirror circuit. We then study the effects of supplying an
explicit object affordance input.
3.5.1.1
Grasp resolution
In Figure 3.13, we let the (trained) model observe a grasp action. Figure 3.13(a) demonstrates the executed grasp by giving the
views from three different angles to show the reader the 3D trajectory
traversed. Figure 3.13(b) shows the extracted hand state (left) and the
response of the (trained) core mirror network (right). In this example, the
network was able to infer the correct grasp without any ambiguity as a single
curve corresponding to the observed grasp reaches a peak and the other two
units’ output are close to zero during the whole action. The horizontal axis
for both figures is such that the onset of the action and the completion of the
grasp are scaled to 0 and 1 respectively. The vertical axis in the hand state
plot represents a normalized (min=0, max=1) value for the components of the
hand state whereas the output plot represents the average firing rate of the
neurons (no firing = 0, maximum firing = 1). The plotting scheme that is used
in Figure 3.13 will be used in later simulation results as well.
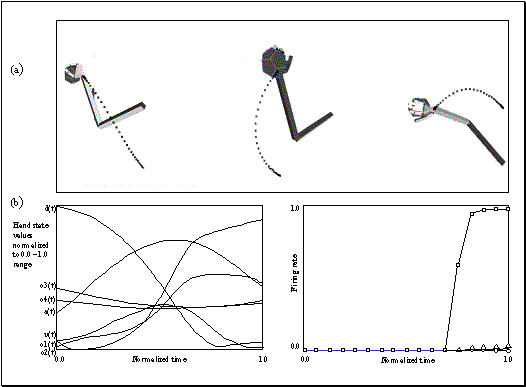
Figure 3.13 (a) A
single grasp trajectory viewed from three different angles to clearly show its
3D pattern. The wrist trajectory during the grasp is marked by square traces,
with the distance between any two consecutive trace marks traveled in equal
time intervals. (b) Left: The
input to the network. Each component of the hand state is labelled. (b) Right: How the network classifies
the action as a power grasp: squares: power grasp output; triangles: precision
grasp; circles: side grasp output. Note that the response for precision and
side grasp is almost zero
It is often
impossible (even for humans) to classify a grasp at a very early phase of the
action. For example, the initial phases of a power grasp and precision grasp
can be very similar. Figure 3.14demonstrates this situation where the model changes
its decision during the action and finally reaches the correct result towards
the end of the action. To create this result we used the "outer
limit" of the precision grasp by having the model perform a precision
grasp for a wide object (using the wide opposition axis). Moreover, the network
had not been trained using this object for precision grasp. In Figure 3.14(b), the curves for power and precision grips cross
towards the end of the action, which shows the change of decision of the
network.
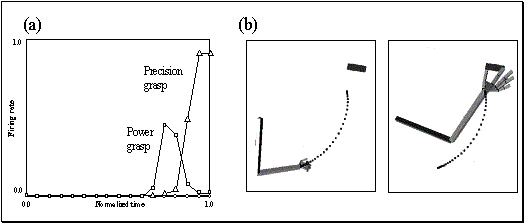
Figure 3.14 Power and precision grasp resolution. The conventions used are as
in the previous figure. (a) The curves for power and precision cross towards
the end of the action showing the change of decision of the network. (b) The
left shows the initial configuration and the right shows the final
configuration of the hand
3.5.1.2
Spatial perturbation
We next analyze how
the model performs if the observed grasp action does not meet the object. Since
we constructed the training set to stress the importance of distance from hand
to object, we expected that network response would decrease with increased
perturbation of target location.
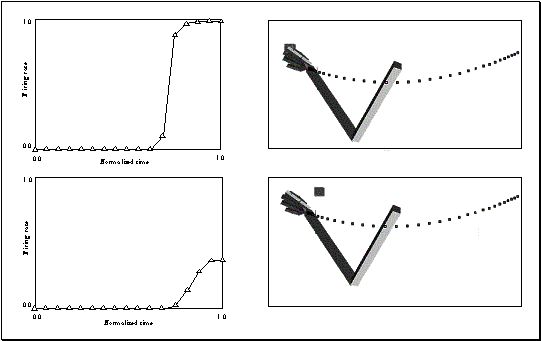
Figure
3.15: (Top) Strong precision grip mirror response for a reaching
movement with a precision pinch. (Bottom) Spatial location perturbation
experiment. The mirror response is greatly reduced when the grasp is not
directed at a target object. (Only the precision grasp related activity is
plotted. The other two outputs are negligible.)
Figure 3.15 shows an example of such a case. However, the
network's performance was not homogeneous over the workspace: for some parts of
the space the network would yield a strong mirror response even with
comparatively large perturbation. This could be due to the small size of the
training set. However, interestingly, the network’s response had some
specificity in terms of the direction of the perturbation. If the object’s
perturbation direction were similar to the direction of hand motion then the
network would be more likely to disregard the perturbation (since the
trajectory prefix would then approximate a prefix of a valid trajectory) and
signal a good grasp. Note that the network reduces its output rate as the
perturbation increases, however the decrease is not linear and after a critical
point it sharply drops to zero. The critical perturbation level also depends on
the position in space.
3.5.1.3
Altered kinematics
Normally, the
simulator produces bell-shaped velocity profiles along the trajectory of the
wrist. In our next experiment, we tested action recognition by the network for
an aberrant trajectory generated with constant arm joint velocities.
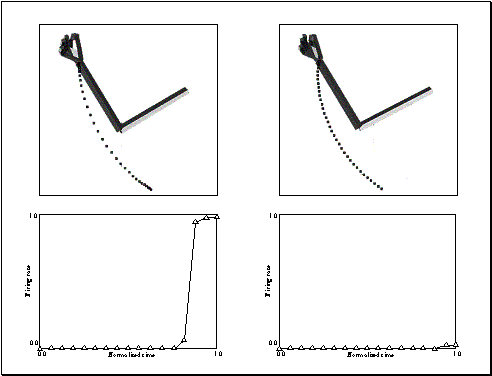
Figure 3.16 Altered kinematics experiment. Left: The simulator executes the
grasp with bell-shaped velocity profile. Right: The simulator executes the same
grasp with constant velocity. Top row shows the graphical representation of the
grasps and the bottom row shows the corresponding output of the network. (Only
the precision grasp related activity is plotted. The other two outputs are
negligible.)
The change in the
kinematics does not change the path generated by the wrist. However, the
trajectory (i.e., time course along the path) is changed and the network is
capable of detecting this change (Figure
3.16). The notable point is that the network acquired this
property without our explicit intervention (i.e. the training set did not
include any negative samples for altered velocity profiles). This is because
the input to the network at any time comprises 30 evenly spaced samples of the
trajectory up to that time. Thus, changes in velocity can change the pattern of
change exhibited across those 30 samples. The extent of this property is again
dependent on spatial location.
It must be stressed
that all the virtual experiments presented in this section used a single
trained network. No new training samples were added to the training set for any
virtual experiment.
3.5.1.4
Grasp and object axes mismatch
The last
virtual experiment we present with non-explicit affordance coding explores the
model’s behavior when the object opposition axis does not match the hand
opposition axis. This example emphasizes that the response of the network is affected
by the opposition axis of the object being grasped. Figure 3.17 shows the axis orientation change for the object and
the effect of this perturbation on the output of the network. The arm simulator
first performed a precision grasp to a thin cylinder. The mirror neuron model’s
response to this action observation is shown in Figure 3.17, leftmost panel. As can be seen from the plot, the
network confidently activated the mirror neuron coding precision grip. The
middle panel shows the output of the network when the object is changed to a
flat plate but the kinematics of the hand is kept the same. The response of the
network declined to almost zero in this case. This is an extreme example – the
objects in Figure 3.17 (rightmost panel) have opposition axes 90° apart,
enabling the network to detect the mismatch between the hand (action) and the
object. With less change in the new axis the network would give a higher
response and, if the opposition axis of the objects were coincident, the
network would respond to both actions (with different levels of confidence
depending on other parameters).
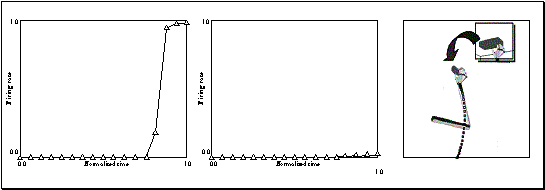
Figure 3.17 Grasp and object axes mismatch experiment. Rightmost: the change of
the object from cylinder to a plate (an object axis change of 90 degrees).
Leftmost: the output of the network before the change (the network turns on the
precision grip mirror neuron). Middle: the output of the network after the
object change. (Only the precision grasp related activity is plotted. The other
two outputs are negligible.)
Now we switch our
attention to the explicit affordance coding network. Here we want to see the
effect of object affordance on the model’s behavior. The new model is similar
to that given before except that it not only has inputs encoding the current
prefix of the hand state trajectory (which includes hand-object relations), but
also has a constant input encoding the relevant affordance of the object under
current scrutiny. Thus, both the training of the network, and the performance
of the trained network will exhibit effects of this additional, affordance,
input.
Due to the simple
nature of the objects studied here, the affordance coding used in the present
study only encodes the object size. In general, one object will have multiple
affordances. The ambiguity then would be solved using extra cues such as the
contextual state of the network. We chose a coarse coding of object size with
10 units. Each unit has a preferred value; the firing of
a unit is determined by the difference of the preferred value and the value
being encoded. The difference is passed through a non-linear decay function by
which the input is limited to the 0 to 1 range (the larger the difference, the
smaller the firing rate). Thus, the explicit affordance coding network
has 220 inputs (210 hand state inputs, plus 10 units coarse coding the size).
The number of hidden layer units was again chosen as 6 and there were again 3
output units, each one corresponding to a recognized grasp.
We have seen that
the MNS model without explicit affordance input displayed a biasing effect of
object size in the Grasp Resolution subsection of Section 5.1; the network was biased
toward power grasp while observing a wide precision pinch grasp (the
network initially responded with a power grasp activity even though the action
was a precision grasp). The model with full affordance replicates the
grasp resolution behavior seen in Figure 3.12. However, we can now go further and ask how the
temporal behavior of the model with explicit affordance coding reflects the
fact that object information is available throughout the action. Intuitively,
one would expect that the object affordance would speed up the grasp resolution
process (which is actually the case, as will be shown in Figure 3.19).
In the following
two subsections we look at the effect of affordance information in two cases:
(i) where we study the response to precision pinch trajectories appropriate to
a range of object sizes; and (ii) where on each trial we use the same
time-varying hand state trajectory but modify the object affordance part of the
input. In each case, we are studying the response of a network that has been
previously trained on a set of normal hand-state trajectories coupled with the
corresponding object affordance (size) encoding.
3.5.2.1
Temporal effects of explicit affordance coding
To observe the
temporal effects of having explicit coding of affordances to the model, we
choose a range of object sizes, and then for each size drive the (previously
trained) network with both affordance (object size) information and the hand-state
trajectory appropriate for a precision pinch grasp appropriate to that size of
object. For each case we looked at the model’s response. Figure 3.18 shows the resultant level of mirror responses for 4
cases (tiny, small, medium, big objects). The filled circles indicate the
precision activity while the empty squares indicate the power grasp related
activity. When the object to be grasped is small, the model turns on the
precision mirror response more quickly and with no ambiguity (Figure 3.18, top two panels). The vertical bar drawn at time 0.6
shows the temporal effect of object size (affordance). The curves representing
the precision grasps are shifted towards the end (time = 1), as the object size
gets bigger. Our interpretation is that the model gained the property of
predicting that a small object is more likely to be grasped with a precision
pinch rather than a power pinch. Thus the larger the object, the more of the
trajectory had to be seen before a confident estimation could be made that it
was indeed leading to a precision pinch. In addition, as we indicated earlier,
the explicit affordance coding network displays the grasp resolution behavior
during the observation of a precision grip being applied to large objects (Figure 3.18, bottom two panels: the graph labeled big object
grasp and to a lesser degree, the graph labeled medium object grasp).
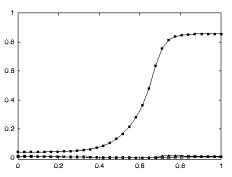
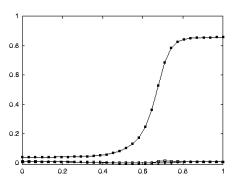
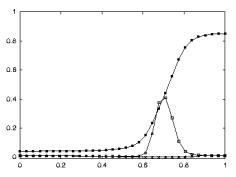
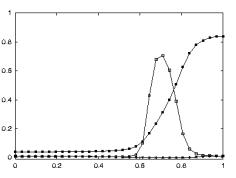
Figure 3.18 The plots show the level of mirror responses of the explicit
affordance coding object for an observed precision pinch for four cases (tiny,
small, medium, big objects). The filled circles indicate the precision activity
while the empty squares indicate the power grasp related activity
We also compared
the general response time of the non-explicit affordance coding implementation
with the explicit coding implementation. The network with affordance input is
faster to respond than the previous one.
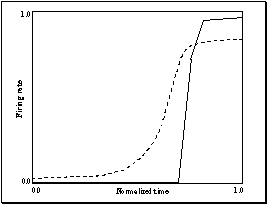
Figure 3.19 The solid curve: the precision grasp output, for the non-explicit
affordance case, directed to a tiny object. The dashed curve: the precision
grasp output of the model to the explicit affordance case, for the same object
Moreover, it
appears that - when
affordance and grasp type are well correlated -
having access to the object affordance from the beginning of the action not
only lets the system make better predictions but also smoothes out the neuron
responses. Figure
3.19 summarizes this: it shows the precision response of
both the explicit and non-explicit affordance case for a tiny object (dashed
and solid curves respectively).
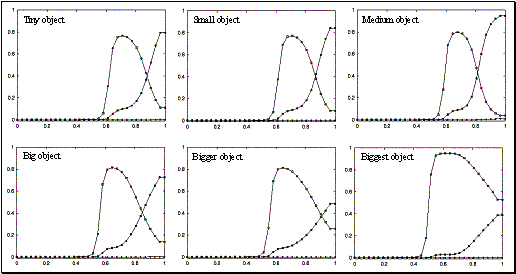
Figure 3.20: Empty squares indicate the precision grasp related cell activity,
while the filled squares represent the power grasp related cell activity. The
grasps show the effect of changing the object affordance, while keeping a
constant hand state trajectory. In each case, the hand-state trajectory
provided to the network is appropriate to the medium-sized object, but the
affordance input to the network encodes the size shown. In the case of the
biggest object affordance, the effect is enough to overwhelm the hand state’s
precision bias.
3.5.2.2
Teasing apart the hand state and object affordance components
We now look at the
case where the hand state trajectory is incompatible with the affordance of the
observed object. In Figure 3.20, the plot labeled medium
object shows the system output for a precision grasp directed to a
medium-sized object whose affordance is supplied to the network. We then
repeatedly input the hand state trajectory generated for this particular action
but in each trial use an object affordance discordant with the observed
trajectory affordance (i.e., using a reduced or increased size of the object).
The plots in Figure 3.20 show the change of the output of the model due to the
change in the affordance. The results shown in these plots tell us two things.
First, the recognition process becomes fuzzier as the object gets bigger
because the larger object sizes biases the network towards the power grasp. In
the extreme case the object affordance can even overwhelm the hand state and
switch the network decision to power grasp (Figure
3.20, graph labeled biggest object). Moreover, for large
objects, the large discrepancy between the observed hand state trajectory and
the size of the objects results in the network converging on a confident
assessment for neither grasp.
Secondly, the
resolution point (the crossing-point of the precision and power curves) shows an
interesting temporal behavior. It may be intuitive to think that as the object
gets smaller the network’s precision decision gets quicker and quicker (similar
to what we have seen in the previous section). However, although this is the
case when the object is changing size from big to small, it is not the case
when the object size is getting medium to tiny (i.e., the crossing time has a
local minimum between the two extreme object sizes, as opposed to being at the
tiny object extreme). Our interpretation is that the network learned an
implicit parameter related to the absolute value of the difference of the hand
aperture and the object size such that the maximum firing is achieved when
the difference is smallest, that is when the hand trajectory matches best with
the object. This will explain why the network has quickest resolution
for a size between the biggest and the smallest sizes.
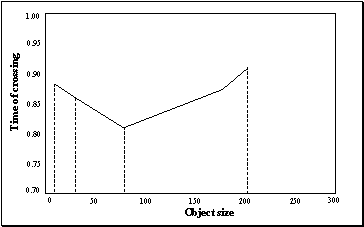
Figure 3.21 The graph is drawn to show the decision switch time versus object
size. The minimum is not at the boundary, that is, the network will detect a
precision pinch quickest with a medium object size. Note that the graph does
not include a point for "Biggest object" since there is no resolution
point in this case (see the final panel of Figure
3.19)
Figure 3.21 shows the time of resolution versus object size in
graphical form. We emphasize that the model easily executes the grasp
recognition task when hand-state trajectory matches object affordance. We do
not include all the results of these control trials, as they are similar to the
cases mentioned in the previous section.
Before closing the
results of this chapter, we would like to present a simulation run using a real
video input to justify our claim that hand state can be extracted from real
video and used to drive the core mirror circuit.

Figure 3.22 The precision grasp action used to test our visual system is
depicted by superimposed frames (not all the frames are shown)
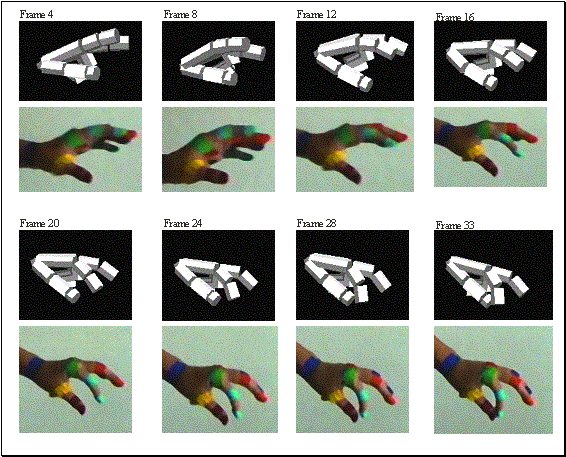
Figure 3.23 The video sequence used to test the visual system is shown together
with the 3D hand matching result (over each frame). Again not all the frames
are shown
The object
affordances are supplied manually as we did not address object recognition in
our visual system. However, the rest of the hand state is extracted by the hand
recognition system as described in Section 3.4.3. Figure
3.22 depicts the precision grasp action used as input
video for the simulation.The result of the 3D hand matching is illustrated in Figure 3.23. The color extraction is performed as described in
the Visual Analysis of Hand State section but not shown in the figure. It would
be very rewarding to perform all our MNS simulations using this system.
However, the quality of the video equipment available and the computational
power requirements did not allow us to collect many grasp examples to train the
core mirror circuit. Nevertheless, we did test the hand state extracted by our
visual system from this real video sequence on the MNS model that has already
been trained with the synthetic grasp examples.
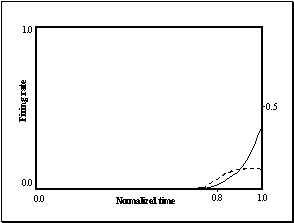
Figure 3.24 The plot shows the output of the MNS model when driven by the
visual recognition system while observing the action depicted in Figure 3.22. It must be emphasized that the training was
performed using the synthetic data from the grasp simulator while testing is
performed using the hand state extracted by the visual system only. Dashed
line: Side grasp related activity; Solid line: Precision grasp related
activity. Power grasp activity is not visible as it coincides with the time
axis
Figure 3.24 shows the recognition result when the actual visual
recognition system provided the hand state based on the real video sequence
shown in Figure 3.23. Although the output of the network did not reach a
high level of confidence for any grasp type, we can clearly see that the
network favored the precision grasp over the side and power grasps. It is also
interesting to note a similar competition (this time between side and precision
grasp outputs) took place as we saw (Figure
3.14) when the grasp action was ambiguous.
Because the mirror
neurons within monkey premotor area F5 fire not only when the monkey performs a
certain class of actions but also when the monkey observes similar actions, it
has been argued that these neurons are crucial for understanding of actions by
others. Indeed, we agree with the importance of this role and indeed have built
upon it elsewhere, as we now briefly discuss. Rizzolatti et al. (1996b) used a
PET study to show that both grasping observation and object prehension yield
highly significant activation in the rostral part of Broca's area (a
significant part of the human language system) as compared to the control
condition of object observation. Moreover, Massimo Matelli (in
Rizzolatti and Arbib 1998)
demonstrated a homology between monkey area F5 and area 45 in the human brain
(Broca's area comprises areas 44 and 45). Such observations led Rizzolatti and
Arbib (1998) building
on Rizzolatti et al. (1996a) to
formulate:
The
Mirror System Hypothesis: Human
Broca’s area contains a mirror system for grasping which is homologous to the
F5 mirror system of monkey, and this provides the evolutionary basis for
language parity - i.e., for an utterance to mean roughly the same for both
speaker and hearer. This adds a neural “missing link” to the tradition that
roots speech in a prior system for communication based on manual gesture.
Arbib (2001) then
refines this hypothesis by showing how evolution might have bridged from an
ancestral mirror system to a ‘language ready’ brain via increasingly
sophisticated mechanisms for imitation of manual gestures as the basis for
similar skills in vocalization and the emergence of protospeech. In some sense,
then, the present paper can be seen as extending these evolutionary concerns
back in time. Our central aim was to give a computational account of the monkey
mirror system by asking (i) What data must the rest of the brain supply to the
mirror system? and (ii) How could the mirror system learn the right
associations between classification of its own movements and the movement of
others? In seeking to ground the answer to (i) in earlier work on the control
of hand movements (Iberall and Arbib 1990) we were led to extend our
evolutionary understanding of the mirror system by offering:
The hand state hypothesis: The basic
functionality of the F5 mirror system is to elaborate the appropriate feedback
– what we call the hand state – for
opposition-space based control of manual grasping of an object. Given this
functionality, the social role of the F5 mirror system in understanding the
actions of others may be seen as an exaptation gained by generalizing from
self-hand to other's-hand.
The hand state
hypothesis provides a new explanation of the evolution of the ‘social
capability’ of mirror neurons, hypothesizing that these neurons first evolved
to augment the ‘canonical’ and ‘pure motor’ F5 neurons by providing visual
feedback on ‘hand state’, relating the shape of the hand to the shape of the
object.
We introduced the
MNS (Mirror Neuron System) model of F5 and related brain regions as an
extension of the FARS model of circuitry for visually-guided grasping of
objects that links parietal area AIP with F5 canonical neurons. The MNS model
diagrammed in Figure 3.5 includes hypotheses as to how different brain regions
may contribute to the functioning of the mirror system. Chapter 6 undertakes
the neural implementation of Grasp Learning (area F4, F2 and F5). This chapter
focused on the Core Mirror Circuit by aggregating the other functionality into
three ‘grand schemas’ -
visual analysis of hand state, reach and grasp. Thus we only claim that core
mirror circuit is relevant for neurophysiological predictions. We developed the
visual analysis of hand state schema to the point of demonstrating algorithms
powerful enough to take actual video input of a hand (though we simplified the
problem by using colored patches) and produce hand state information. The reach
and grasp schema then represented all the functionality for taking the location
and affordance of an object and determining the motion of a hand and arm to
grasp it (however see Chapter 6 for a detailed neural implementation of this
circuit grounded in neurophysiology and infant behavior). As the main aim of
this chapter was to analyse the core mirror circuit we showed that if we used
the reach and grasp schema to generate an observed arm-hand trajectory (i.e.,
to represent the reach and grasp generator of the monkey or human being
observed), then that simulation could directly supply the corresponding
hand-state trajectory, and we thus use these data so that we can analyze the
core mirror circuit schema (Figure
3.6(b)) in isolation from the visual analysis of hand state.
However note that we have also justified the visual analysis of hand state
schema by showing in a simulation that the core mirror circuit can be driven
with the proposed vision system without any synthetic data from the reach and
grasp schema.
Moreover, the hand
state input (regardless of being synthetic or real) was presented to the
network in a way to avoid the use of a dynamic neural network. To form the
input vector, each of the seven components of the hand state trajectory, up to
the present time t, is fitted by a cubic spline. Then this spline is sampled at
30 uniformly spaced intervals; i.e., no matter what fraction t is of the total
time T of the entire trajectory, the input to the network at time t comprises
30 samples of the hand-state uniformly distributed over the interval [0, t].
The network is trained using the full trajectory of the hand state in a
specific grasp; the training set pairs each such hand state history as input
with the final grasp type as output. On the contrary, when testing the model with
various grasp observations, the input to the network was the hand state
trajectory that was available up to that instant. This exactly parallels the
way the biological system (the monkey) receives visual (object and hand)
information: When the monkey performs a grasp, the learning can take place
after the observation of the complete (self) generated visual stimuli. On the
other hand, in the observation case the monkey mirror system predicts the grasp
action based on the partial visual stimuli (i.e. before the grasp is
completed). The network thus yields a time-course of activation for the
mirror neurons, yielding predictions for neurophysiological experiments by
highlighting the importance of the timing
of mirror neuron activity. We saw that initial prefixes will yield little
or no mirror neuron activity, and ambiguous prefixes may yield transient
activity of the ‘wrong’ mirror neurons.
Since our aim was
to show that the connectivity of mirror neuron circuitry can be established
through training, and that the resultant network can exhibit a range of novel,
physiologically interesting, behaviors during the process of action
recognition, the actual choice of training procedure is purely a matter of
computational convenience, and the fact that the method chosen, namely
back-propagation, is non-physiological does not weaken the importance of our
predictions concerning the timing of
mirror neuron activity.
With this we turn
to neurophysiological predictions made in our treatment of the Core Mirror
Circuit, namely the ‘grounding assumptions’ concerning the nature of the input
patterns received by the circuit and the actual predictions on the timing of
mirror neuron activity yielded by our simulations.
Grounding assumptions: The key
to the MNS model is the notion of hand
state as encompassing data required to determine whether the motion and
preshape of a moving hand may be extrapolated to culminate in a grasp
appropriate to one of the affordances of the observed object. Basically a
mirror neuron must fire if the preshaping of the hand conforms to the grasp
type with which the neuron is associated; and the extrapolation of hand state yields a time at which the
hand is grasping the object along an axis for which that affordance is
appropriate. What we emphasize here is not
the specific decomposition of the hand state F(t) into the seven specific components (d(t), v(t), a(t), o1(t), o2(t), o3(t),
o4(t)) used in our simulation, but rather that the input neural
activity will be a distributed neural code which carries information about the
movement of the hand toward the object, the separation of the virtual
fingertips and the orientation of different components of the hand relative to
the opposition axis in the object. The further claim is that this code will
work just as well for measuring how well another
monkey’s hand is moving to grasp an object as for observing how the monkey’s
own hand is moving to grasp the object, allowing self-observation by the monkey
to train a system that can be used for observing the actions of others and
recognizing just what those actions are.
We provided experiments to compare the performance of the
Core Mirror Circuit with and without the availability of explicit affordance
information (in this case the size of the object) to strengthen our claim that
it is indeed adaptive for the system to have this additional input available,
as shown in Figure 3.6(b). Note that the "grasp command" input
shown in the figure serves here as a training input, and will, of course, plays
no role in the recognition of actions performed by others.
Also we have given a justification of the visual analysis of
hand state schema by showing in a simulation that the core mirror circuit can
be driven with the visual system we implemented without requiring the Reach and
Grasp simulator provide syntetic data.
Novel Predictions: Experimental
work to date tends to emphasize the actions to be correlated with the activity
of each individual mirror neuron, while paying little attention to the temporal
dynamics of mirror neuron response. By contrast, our simulations make explicit
predictions on how a given (hand state trajectory, affordance) pair will drive
the time course of mirror neuron activity – with non-trivial response possibly
involving activity of other mirror neurons in addition to those associated with
the actual grasp being observed. For example, a grasp with an ambiguous prefix
may drive the mirror neurons in such a way that the system will, in certain
circumstances, at first give weight to the wrong classification, with only the
late stages of the trajectory sufficing for the incorrect mirror neuron to be
vanquished.
To obtain this prediction we created a scene where the
observed action consisted of grasping a wide object with precision pinch (thumb
and index finger opposing each other). Usually this grasp is applied to small
objects (imagine grasping a pen along its long axis versus grasping it along
its thin center axis). The mirror response we got from our core mirror circuit
was interesting. First, the system recognized (while the action was taking
place) the action as power grasp
(which is characterized by enclosing the hand over large objects; e.g. grasping
an apple) but as the action progressed the model unit representing precision
pinch started to get active and the power grasp activity started to decline.
Eventually the core mirror circuit settled on the precision pinch. This
particular prediction is testable and indeed suggests a whole class of
experiments. The monkey has to be presented with unusual or ambiguous grasp actions
that require a ‘grasp resolution’. For example, the experimenter can grasp a
section of banana using precision pinch from its long axis. Then we would
expect to see activity from power grasp related mirror cells followed by a
decrease of that activity accompanied by increasing activity from precision
pinch related mirror cells.
The other simulations we made leads to different testable
predictions such as the mirror response in case of a spatial perturbation
(showing the monkey a fake grasp where the hand does not really meet the
object) and altered kinematics (perform the grasp with different kinematics
than usual). The former is in particular a justification of the model, since in
the mirror neuron literature it has been reported that the spatial contact of
the hand and the object is usually required for the mirror response (Gallese et al. 1996). On the other hand, the
altered kinematics result predicts that an alteration of the kinematics will
cause a decrease in the mirror response. We have also noted how a discrepancy
between hand state trajectory and object affordance may block or delay the
system from classifying the observed movement.
In summary, we have conducted a range of simulation
experiments – on grasp resolution, spatial perturbation, altered kinematics,
temporal effects of explicit affordance coding, and analysis of compatibility
of the hand state to object affordance – which demonstrate that the present
model is not only of value in providing an implemented high-level view of the
logic of the mirror system, but also serves to provide interesting predictions
ripe for neurophysiological testing, as well as suggesting new questions to ask
when designing experiments on the mirror system.
This chapter introduces a learning and data generation model
that can be employed in multi-layered circuits. The architecture that we develop
in this chapter will be used in the Grasp Learning Models of Chapters 5 and 6.
The adaptation of the network weights is performed in a hebbian fashion based
on a reinforcement signal. In the general reinforcement learning framework the
learning problem is formulated as an agent acting in an environment that
returns rewards based on the actions of the agent and state of the environment (Sutton and Barto 1998). By acting, the agent can
(and usually does) change the state of the environment. The goal of the agent
is to maximize its total reward in the long run, possibly in infinite future (Sutton and Barto 1998). Unlike other optimization
based learning methods, reinforcement learning can be implemented by biological
circuits. The supervised Hebbian learning that we introduce in this chapter is
a special case of the general reinforcement learning. We will use the terms
supervised Hebbian and reinforcement learning interchangeably.
Although there is no agreement on the exact coding neurons
employ in the brain we in general adopt a population coding approach where
information is represented in the activities of a group of neurons. What we
mean by the activity of a neuron is the average firing rate. We do not reject
other possibilities such as interval coding where the timing of the spikes carry
information but we adopt average firing rate. There is large amount of
experimental data showing that a populating coding scheme is employed in the
brain (e.g. see Georgopoulos
1986).
The term preferred-stimulus is used to indicate the stimulus that causes a
neuron to fire maximally. It is observed that neurons would fire for the
stimuli which are not the best, with a decreasing rate as the stimuli
diverges from the preferred one. In most cases, the population code is thought
to be encoding a single variable (like the orientation of an edge)(Zemel et al. 1998). However, the population can
be involved in encoding more than the value of a variable such as the variance
and uncertainty of the variable (Zemel et al. 1998). Indeed there are cases where
the probability distributions of the variables are more adequate than the
single values as when insufficient information exists to differentiate between
two values of the variable (stimuli), or when multiple values underlie the
input (former case, place cells in the hippocampus (O'Keefe and Dostrovsky
1971);
the latter case directional motion detecting cells in MT (Newsome et al. 1989)).
We adapt the probabilistic interpretation of the population
coding (Anderson 1994; Zemel et
al. 1998)
to represent multiple values or options given the input stimuli.
For example, given a sphere shaped object, what is the most natural
representation of a parameter that specifies the direction (e.g. top, down,
right, etc.) from which the animal should grasp the object? The most general
answer to this question is to have a representation to encode the parameter with
its probability distribution. Indeed, in this example, the sphere can be
grasped in very many ways. We view each layer in a network as representing the
possible choices for the next layer. When the animal needs to act based on the
processed input then it samples over the output to generate the movement
possibly by a winner-take-all mechanism.
The ‘usual’ way of using a neural network to perform density
estimation is much like a function approximation, where the network is asked to
give a single output (the value of the probability density function) given an
input (see Bishop 1995). In the architecture we
proposed, a neural structure is used to compute a normalized histogram in terms
of population activity. Besides biological relevance, by representing the
distribution as a population code, we gain the power to use the network’s
output as inputs to other networks or layers without an extra decoding step.
In the abstract setting, we posit an agent that takes actions
and the environment (can include the agent itself) returns rewards or binary
signals indicating whether the action was ‘good’ or ‘bad’. Some information of
the environment may be available to the agent as input. We denote the output of
the agent with y(t), the input as x(t) and the
reward returned by r(t). We are interested in constructing a network
that can be implemented using neural units to achieve: (1) estimation of the
probability distribution of the reward given an input, and (2) generating
outputs that return rewards approximating the probability distribution of the
reward.
We will use X(t) to denote the input instead of the x(t)
to emphasize that in general the input can be the output of a different layer.
A layer is composed stochastic units (Hertz et al. 1991). The layer generates an
output based on the probability distribution represented by Y(t), the
firing potentials of the stochastic units (described below). We introduce
layers Xe, Ye as memory traces (a rudimentary form of
the 'eligibility traces' of Sutton and Barto 1998) for keeping the memory of
activities of layers X and Y, respectively (we will use ‘memory
trace’ and ‘eligibility’ terms interchangeably in this chapter). The
connectivity between layers X and Y is established through the
weight matrix W. The network operates as the following:
- Input
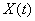 is presented
is presented
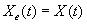
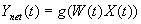
where 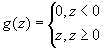
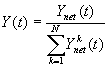
- For k=1,..,N
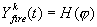 with probability
Yk(t),
where j
non-negative small (<<1) uniform noise term and H is the step
function
with probability
Yk(t),
where j
non-negative small (<<1) uniform noise term and H is the step
function
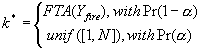 where FTA
stands for First-Fire Take All process
where FTA
stands for First-Fire Take All process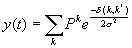 , where Pk
is the preferred action associated with unit k and s2
is the variance determining the size of the weighted sum range
, where Pk
is the preferred action associated with unit k and s2
is the variance determining the size of the weighted sum range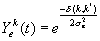 , for k=1,..,N
, for k=1,..,N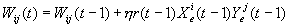 , where h is the learning rate
parameter
, where h is the learning rate
parameter
The steps 1-2, is standard: the input is propagated via a
weight matrix to form the net input. Step 3 applies a threshold operation to
eliminate negative net inputs. Step 4 converts the net input into firing
potentials by normalization. The function of steps 5 and 6 is not as apparent.
Each stochastic unit fires with probability that is equal to its firing
potential (computed in Step 4). However, the spike generation timing is
variable due to uniform noise (Step 5). Step 6 has two modes of operation. With
probability a,
a random unit is selected from Y as a winner (k*), and with probability
1-a,
a FirstFire-Take-All
process is applied on the stochastic units to find real winner (k*).
Let us consider the case where no exploratory moves are
performed (a=0),
then what is the distribution of k*? The Steps 5 and 6 generate winner
k*s such that 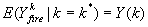 holds for all k.
In essence we implement an approximate universal data generation (see Leydold and Hormann
2000 for a review)
method that is open to neural implementation. Step 7 implements a local
population vector computation around the winning unit. The distance metric d
defines how similar the neurons’ preferred values are. This metric imposes a
topological relation between the units through the learning rule. Step 8
creates eligibility traces to register the activated regions for the next
cycles weight update. Step 9 updates the weights in a supervised Hebbian
learning fashion (or reinforcement learning update as in Willams’ (1992) REINFORCE). The weight update
rule creates a normalized histogram of the reward yielding input patterns: Claim:
Given input Xm , one of the S distinct input vectors,
at time step p, Y(k) approximates the probability distribution of the
reward. Proof: We are given the distinct input patterns as (X1,
X2, .., Xs). We prove the claim with
the following assumptions:
holds for all k.
In essence we implement an approximate universal data generation (see Leydold and Hormann
2000 for a review)
method that is open to neural implementation. Step 7 implements a local
population vector computation around the winning unit. The distance metric d
defines how similar the neurons’ preferred values are. This metric imposes a
topological relation between the units through the learning rule. Step 8
creates eligibility traces to register the activated regions for the next
cycles weight update. Step 9 updates the weights in a supervised Hebbian
learning fashion (or reinforcement learning update as in Willams’ (1992) REINFORCE). The weight update
rule creates a normalized histogram of the reward yielding input patterns: Claim:
Given input Xm , one of the S distinct input vectors,
at time step p, Y(k) approximates the probability distribution of the
reward. Proof: We are given the distinct input patterns as (X1,
X2, .., Xs). We prove the claim with
the following assumptions:
- Memory trace of Y is concentrated on a single
point (let Z(t)=Ye(t) for notional convenience).
- The rewards returned by the environment are
non-negative
- Number of the training inputs is far larger than the
number of distinct input patterns (p>>S)
- a=1
Given a randomly initialized network, When we apply the
inputs X0, X1, X2 …, Xp-1
according to the layer update equations (1-9) above we get: (the subscripts
denote the time step while the superscripts denote the pattern number.)
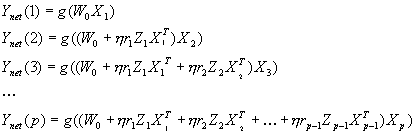
Noting that the argument of g is nonnegative and the initial
weights’ (W0) contribution can be made arbitrarily small we
can write:
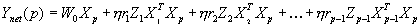
If the input patterns that generate the rewards are
approximately mutually orthogonal then we can write:
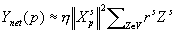 where s is from
1,..,S (s indexes the distinct input patterns)
where s is from
1,..,S (s indexes the distinct input patterns)
Here, V is the set of Z’s that appear with Xs in the expression for Ynet(p).
Therefore Ynet(p) is the total reward for each Z
in V. If the reward returns are binary, then Ynet(p)
becomes (unnormalized) histogram of the occurrence of rewards. Thus, step 4 generates the normalized
histogram.
In simulations, we observed that mild violation of
the assumptions used in the proof does deform the final representation in Y. However, the value of a
requires some comment. This parameter controls how much the layer is exploring
as opposed to exploiting the current probability distribution. The learning
rate, h,
should be reduced as the layer starts exploiting more. When h and a are
chosen constant non-zero values, in the limit, the layer degenerates into a
winner-take-all circuit. To see this observe that in the expression of Ynet(p)
each presentation of input is accompanied by a Z value which is the
indicator of the unit (and its neighborhood) that determined the action.
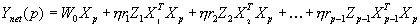
When a=0, Z does not depend on
the current state of the layer. If a>0 then the action generation
will pick actions from a neighborhood of the actions that returned reward (with
probability a).
That is the terms of Ynet(p) will be biased. Thus, the
histogram computed by the layer will be sharper. In practice, having small a (a<0.2),
reflects the reward distribution in the layer and provides enough exploitation
that the learning is faster than trial and error (i.e. a=0 case). The Hebbian
update we use in Step 9 although, biologically realistic, does not limit the
weight growth. In practice, either a weight normalization or use of an adaptive
learning rate that tends to zero is required if the weights need to be bounded.
The analysis when the rewards
can be negative is similar. A non-zero Ynet(P) can be split
into negative and positive reward terms and written in this form:
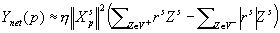 where V+ is the set of Z’s
that appear with Xk for which rk>0 and V-
is the set of Z’s that appear with Xk for which
rk<=0.
This simply tells us that given an input Xs at time step p, the
firing potential is represented as the difference of two distributions: the
positive reward distribution and the negative reward distribution. An intuitive example would be the situation
where an action of the agent returns +1 or –1 with equal probability
(regardless of state of the environment). Then the learning will generate a Ynet
distribution where the action is represented as returning 0 reward.
where V+ is the set of Z’s
that appear with Xk for which rk>0 and V-
is the set of Z’s that appear with Xk for which
rk<=0.
This simply tells us that given an input Xs at time step p, the
firing potential is represented as the difference of two distributions: the
positive reward distribution and the negative reward distribution. An intuitive example would be the situation
where an action of the agent returns +1 or –1 with equal probability
(regardless of state of the environment). Then the learning will generate a Ynet
distribution where the action is represented as returning 0 reward.
We present two example problems to demonstrate that the
architecture we proposed can learn to generate actions that capture the
distribution of the reward. In both cases, we take an environment with known
reward distribution and we let the agent interact with the environment with the
learning rules specified in the previous sections. After the training, we check
whether, by interacting with the environment, the network was able to discover
the underlying distribution.
In the next chapter we will use this architecture for
multivariate action domains, so we chose a two-dimensional action space to make
sure that the architecture is adequate for the grasp learning tasks of the next
chapters.
The test problem is defined as the following. The agent acts
by generating a vector (x, y) where each component is from the interval [ –1,
1]. Then the environment returns a deterministic reward of +1 if (x*x+y*y)
<0.25 and –1 otherwise (Figure
4.1). We used 400 (20x20 grid) neurons to represent the
output in the layer. The topology of the layer is reflected through the
specification of the distance metric, d. We defined the distance
metric as the usual Euclidean metric over the 2D action space.
Figure 4.1 The
elevated circular region corresponds to the area defined by the equation
(x*x+y*y) <0.25. The environment returns +1 as the reward if the action
falls into the circular region, otherwise –1 is returned.
In the learning
simulations, the agent generated the actions from its action probability
distribution (Y) with 0.5 probability and performed exploratory trials
with 0.5 probability
(i.e. a=0.5).
Figure 4.2 shows the evolution of the network outputs (Y)
subject to the learning rules specified in the previous sections. The firing
potential (Y) is shown as a grid to allow comparison with the
environment distribution.
Figure 4.2 The
adaptation of the firing potential of the stochastic units are shown as a
series of evolving 3D maps. (left to right and top to bottom)
Figure 4.2 only confirms that the firing potentials of the
output units capture the reward distribution. We have to test our assertion
that the generation of actions by using Steps 5 and 6 of the previous section
approximates the distribution of the environment. Figure 4.3, shows that the actions generated with the dynamics
we proposed in the previous section leads to a histogram that approximates the
environment’s reward distribution (compare to Figure
4.1).
Figure 4.3 The
normalized histogram of the actions generated over 60000 samples. Note that the actions
generated captured the environment’s reward distribution (see Figure 4.1).
The environment we defined above was simple: it was
deterministic and the set of actions that would yield reward was convex. Now we
confront the architecture we proposed with a more realistic case, where the
reward distribution is multimodal and the rewards are returned stochastically.
Given an action (x, y) the environment returns a reward of +1
with the probability:
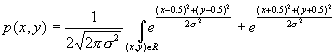 ,
,
where s2 = 0.1 and R is
the rectangular grid of size 0.05x0.05 where (x,y) falls in.
The distribution is the summation of two Gaussian centered
on (0.5, 0.5) and (-0.5,-0.5), hence double peaked. (Figure 4.4)
Figure 4.4 The
stochastic environment’s double peaked reward distribution (see text for the
explanation)
Figure 4.5 Some snapshots showing the phases of
learning of the layer in the stochastic environment where the reward
distribution has two peaks (see Figure
4.4).
The evolution of the layer’s firing potential presented in Figure 4.4 shows differential phases than the unimodal
deterministic environment. First, the peaks are sorted out as isolated sharp
peaks with different heights. Then the potentials settle on equal height peaks
capturing the input distribution (Figure
4.4).
Similar to what we did in the deterministic environment
case, we generated actions using the trained layer to test whether the
distribution of the generated action matches the input reward distribution. Figure 4.6 shows the normalized histogram of the actions
generated. The main structure of the environment was captured (compare to Figure 4.4).
Figure 4.6. The
normalized histogram of 60000 data points (actions)
generated by the trained layer in the stochastic environment depicted in Figure 4.4.
The main aim of introducing the proposed architecture is to
combine them for more complex computations. In a hypothetical problem, layers A
and B independently compute some parameters. A third layer, layer C uses the
output of layer A and B to generate the final output of the combined network.
The output of C depends on both A and B. Therefore C has to discovered the
action distribution give the inputs from A and C. The combining layer performs
this via a synaptic multiplication (we drop the time dependency and use matrix
notation for clarity). The subscript e is used to denote the eligibility traces
as before. The synaptic input channels of C and the corresponding weights are
denoted with CsynA, CsynB; and WsynA,
WsynB
Compute the net synaptic inputs:
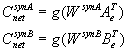 where
where 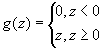
Compute the normalized synaptic inputs:
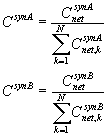
Combine the normalized synaptic inputs:
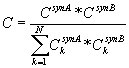
The action generation from layer C, is the same is in the
single layer case (Steps 5-8). The synaptic weight update is based on the
eligibility of layers. The parameter l denotes the learning
rate for the synaptic connections.
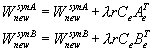 , s
, s
Chapters 5 and 6 will present the learning to grasp models.
The architecture described here will be used as the mechanism of learning and
grasp plan generation. We avoid non-biological
computations in the architecture presented here so that we can make realistic
prediction with models that use this architecture (Chapter 6). The weight
adaptation that is offered in this chapter is based on reinforcement signal and
thus biologically plausible. The parameters are represented as local population
vectors and hence are open to neurophysiological predictions.
The layer structure we proposed, in essence, computes a
histogram of the input patterns that yield rewards. The action that brings
reward causes a weight strengthening between the eligibility trace of the unit
coding the input stimuli and the eligibility trace of the neuron coding the
action. The output trace left is broader than the input (Step 8). This way the
weight strengthening not only increases the chance of the neuron that coded the
rewarding output to become active next time but also it increases the chance of
neighboring output units becoming active as well.
We introduced a neural network architecture composed of
stochastic units with the following working principle. Each stochastic unit
receives a net input that is the value after the input vector is passed
through a linear transformation (weight multiplication) followed by a threshold
operation. Then the units belonging to the same layer are normalized so that
their activity sums up to 1. The normalized value of each unit is called the
units firing potential. Then each unit fires stochastically according to
its firing potential. However, the firing timing is variable and the
unit which fires first (in a given a cycle period) suppress its peers (becomes the winner). The value
output from the layer is computed as a local population vector summation. The
memory traces of which unit fired is kept for the next cycle when the reward
signal would be available for updating the weights. If the output generated
returned positive reward then the connections that contributed to the firing
are enhanced; if the output was a negative reinforcement then the weight that
contributed to the output generation are reduced.
This simple reinforcement learning based layer is able to
represent the probability distribution of reward conditioned on the input. A
very intuitive way to look at the network is to notice that each unit is like a
counter, counting how many times it is involved in positive-reward
situations. The negative weights, the threshold operation and variable reward
(i.e. non binary) makes the mathematical analysis nontrivial. Nevertheless, we
presented some proofs with certain assumptions and showed the architecture
works as we described.
The network is based on biologically realistic computations.
Reinforcement learning, population coding, lateral inhibition/shunting (for
FTA) and stochastic spike generation (based on the firing potential) are
biologically feasible computations. This will enable us to use the architecture
in Grasp Learning Model (LGM) of Chapter 6.
In next chapters we will combine layers, each of which is a
network of the type we presented here to develop more complex networks. In
Chapter 5 we use the multilayer network for infant grasp learning (ILGM).
Chapter 6 instantiates the Chapter 5’s multilayer grasp learning model as a
network of brain regions with respect to monkey neurophysiology and
neuroanatomy, which enables us to perform neural level analyzes yielding to
neurophysiological predictions.
This chapter presents a computational model of infant grasp
learning constrained by infant motor development studies. Key elements of the
infancy period, namely elemental motor schemas, the explorative nature of
infant motor interaction and inherent motor variability are captured in the
model to produce testable predictions and explain how an existing behavior
(reaching) yields a more complex behavior (grasping) through exploratory
learning.
Many research fields focus on discovering the principles of
limb movement control. Neural network researchers address problems of internal
representation, learning and execution; Roboticists solve the problems of
kinematics and dynamics (Arbib and Hoff 1994) and Computational
neuroscientists try to understand principles how the biological systems manage
motor control. From a computational point of view, once the fingers to use
and/or the wrist position, and the targets of the fingers on the object, are
specified kinematics of grasping can be formulated using inverse kinematics
methods (Sciavicco and Siciliano
2000).
Inverse kinematics methods are based on reducing various kinematics error
functions (readers are referred to Arbib and Hoff (1994) for a review of models of
reach to grasp).
Although it has been suggested that human grasping involves
separate control of digits so as to bring the fingers to their targets as in
inverse kinematics methods (Smeets and Brenner
2001; Smeets and Brenner 1999), the general view is that hand transport and
finger movements are controlled separately (see Jeannerod et al.
1998).
We used the former approach when we studied the Mirror Neuron System Model
(MNS) for generating grasp actions to provide visual input stimuli for the MNS
model (Chapter 3). However, we will take a very different approach for infant
grasp learning.
From the viewpoint of an infant, what does ‘error’ mean? How
is the finger-to-target assignment made? These are the questions that motivate
our modeling study of infant grasp learning. This Chapter is built on the
notion that infants initially neither have a concept of minimizing ‘error’ nor
know how to match their hands to objects for grasping; however, they do sense
the effects of their motor acts which enables them to adjust their behaviors.
A child learns its own possibilities of action in the
environment (affordances) through exploratory behavior (Jeannerod et al. 1998). By 2-3 months, infants start
exploring their bodies as they move in the environment, they babble and touch
their own body. They are actively involved in investigating the intermodal
redundancies, temporal and spatial relations of their self-perception (Smeets and Brenner
2001).
As new skills are acquired, new action domains are opened for the infant.
Infants progress from a crude ability of reaching at birth to finer reaching
and further grasping ability around four months of age. Infants learn to
overcome problems associated with reaching and grasping by interactive searching
(Berthier et al. 1996;
von Hofsten 1993).
The precision grasp appears around 12-18 months of age (Berthier et al. 1999).
To grasp successfully infants have to learn how to control
their arms and, further, to match the abilities of their limbs with affordances
presented by the environment (Bernstein 1967; Gibson
1969; Gibson 1988; Thelen 2000). Our study focuses on the latter problem; that
is discovering how to generate grasps that match the affordances presented by
the objects in the environment. Rochat and Morgan (1995) have shown that infants are
aware of visual, proprioceptive and haptic consequences of their limb
movements.
The onset of reaching and grasping marks a significant achievement
in infants’ functional interactions with their surroundings. The advent of
voluntary grasping of objects is preceded by several weeks in which the infant
engages in arm movements and fisted swipes in the presence of visible objects (von Hofsten 1984). The term visually guided reaching generally
refers to the infants having available continuous vision of the hand and
target, whereas visually elicited
reaching refers to the vision of the target, followed by a ballistic hand
movement. Clifton et al. (1993) questioned the hypothesis
that the earliest accurate reaching behavior is visually guided and appears
around 3-5 months (Clifton et al. 1993). They tested seven infants
repeatedly between 6 and 25 weeks of age using glowing objects in the light
condition and sounding objects for the dark condition. Infants first contacted
the object in both dark and light conditions at almost the same ages (mean
ages: 12.3 weeks for light, 11.9 weeks for dark condition). Infants first
grasped the object in light condition at 16.0 weeks and in the dark at 14.7
weeks. Even though this was not a statistically significant difference, there
is other evidence that the self-vision of the hand may retard successful grasp
in infants younger than 5 months of age. This could be due to the attentional
load that is brought by the existence of two objects in the visual field (Streri 1993). Clifton et al. (1993) argued that since infants
could not see their hand or arm in the dark, their early success in contacting
the glowing and sounding objects indicated that proprioceptive cues, not sight
of the limb, guided their early reaching. This shows that early reaching (and
grasping –evidenced in the following text) is performed using an open-loop
strategy, because it appears that, the initial localization (glow or the sound
of the object) was enough to perform successful movement planning. Reaching in
the light developed in parallel with reaching in the dark, suggesting that
visual guidance of the hand is not necessary to achieve object contact. It is
also noteworthy that infants showed great individual differences. Onset for
touch varied between 7 and 16 weeks, while onset for grasp varied between 11
and 19 weeks. The greatest discrepancy (light versus dark conditions) in onset
of reach and grasp was 4 weeks. There were three infants out of seven with this
discrepancy. Interestingly for all the three infants the behavior occurred
earlier in the dark.
An infant, once contacted an object, will occasionally try
to grasp it (Clifton et al. 1993). The enclosure reflex
disappears around six moths of age and it takes four more weeks of infancy to
stabilize the grasp (Clifton et al. 1993). It is suggested that the
fractionated control of finger movements is not possible since this task
requires the cortico-motoneuronal system, which has not fully developed at this
age. (Lantz et al. 1996). Before nine months of age,
the infant grasp lacks the anticipation of the orientation and size of the
object (Rosenbaum 1991). Infants adjust their grasps
after touching the objects. In contrast, adults adjust their distance between
the thumb and the other fingers according to the size of the object during the
hand transport. This holds even though infants younger than nine months old are
physically able to vary their grip size, for they can spread their fingers
farther apart once they have felt a large object (von Hofsten and
Ronnqvist 1988).
It appears that in early infancy the fractionated control of fingers is mainly
driven by somatosensory feedback.
Butterworth et al. (1997) studied the development of
prehension in infants by video recording the grasping behavior of babies from 6
to 20 months of age using objects of different shapes and different sizes. The
infants were divided into four groups according to their age. The objects used
were wooden cubes and spheres with sizes of 0.5cm, 1cm, 2cm and 3cm.
Butterworth et al. (1997) classified the grips used in the grasps according to
two broad categories of power and precision grips. The power grip had
subdivisions of ulnar grasp, hand grasp, palm grasp and radial palm grasp while
the precision grip had subdivisions of scissor grasp, inferior forefinger
grasp, inferior pincer grasp and pincer grasp (Figure 5.1). The main result of this study was that both power
and precision grips were observed in infants older than 6 months of age and
there is a developmental trend such that in the early second year the
occurrence of power grips decreased while the occurrence of precision grips
increased, and eventually became the dominant mode of prehension. Furthermore,
the size rather than shape (cube vs. sphere) determined the grasp type (Butterworth et al.
1997).
The younger infants did not employ a consistent pattern of grasping for small
objects whereas the older infants developed preferential grasp patterns for
certain sizes (0.5 cm, pincer grasp; 2 cm, forefinger grasp) (Butterworth et al.
1997).
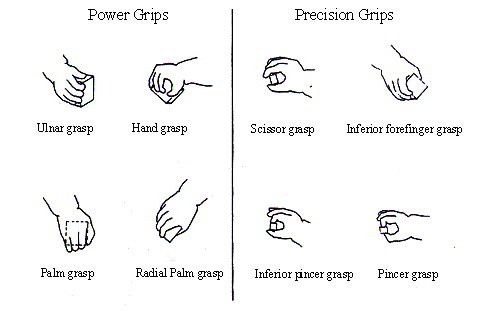
Figure 5.1 Infant grip configurations
can be divided in two categories: power and precision grips. Infants tend to
switch from power grips to precision grips as they grow (adapted from Butterworth et
al. 1997)
Lockman et al. (1984) investigated the development
of infants’ prehensile adjustments regarding the orientation of objects. They
compared the performance of groups of 5 and 9 months old infants (8 infants in
each group). The infants were presented wit a dowel with two orientations,
vertical and horizontal. In the trials, if the infant did not initiate a grasp
when the dowel is revealed, the experimenter attracted the attention of the
infant to encourage him/her to grasp the dowel. If the infant did not grasp but
touched the dowel, the trial was terminated and excluded from the analysis. The
hand orientations were analyzed by recording the orientations at four points in
time: 1) at the beginning (with the first forward movement of the hand); 2)
When the hand passed the midpoint between the first point and the object
position; 3) at the time of first contact of the hand with the dowel; 4) and at
the end where at least one digit closed around the dowel. The principal finding
was that 9 months old infants oriented their hands appropriately earlier during
the reach to grasp than did 5 months old infants. Furthermore, the two age
groups differed at the last two stages of the grasp (touch and enclosure)
whereas the earlier parts of their reaches were similar.
In another related study, 102 infants between 4-8 months old
were grouped according to their age and was studied their grasping behavior as
a function of object shape and size using seven combinations of objects (Newell et al. 1989). The main finding of this
study is that the youngest age group (4 months) required the addition of the
haptic system for successful grasp whereas the oldest age group (8 months)
mainly relied on information from the visual system to differentiate grip
configuration according to the object properties. They found that there was a
remarkable similarity between the grasp configurations achieved, irrespective
of whether they are visually planned or haptically adjusted.
Butterworth et al. (1997) show that the young infants
display a wide variety of grip types that were conventionally not attributed to
6-8 months old infants (Halverson 1931),which is in agreement with
our simulations. Lockman et al. (1984) used a single object with
different orientations in such a way that the infant’s inability to plan
according to the target can be detected. An inappropriate hand configuration
would cause a big discrepancy between the hand orientation and the object
orientation.
Finally, Newell et al. (1989) inform us that the older
infants’ visually programmed and younger infants’ haptically adjusted grasp
configurations are very similar. This strongly suggests that the earlier haptic
grasping phase serves as the training stimuli for visual grasp planning circuit
in infant brain. We use the reviewed studies to constrain and design the
Learning to Grasp Model, and evaluate its relevance to infant learning through
explicit comparisons.
The corticospinal tract is one of the main neural substrate
for independent finger control (Triggs et al. 1998): Firstly, corticospinal
projections terminating in the ventral horn innervating hand muscles predict
independent finger use for small objects (Bortoff and Strick
1993).
Secondly, lesioning the corticospinal tract prevents the development of the
independent finger movement in infant animals (Lawrence and Hopkins
1976),
and impairs independent finger movements. Human patients recovering from
corticospinal lesions initially tend to perform synergistic movement of all the
fingers as in a power grip (Denny-Brown 1950;
Lassek 1954).
Given these data, it is tempting to theorize that infants’
late grasping development is due to neural maturation. This view is embraced by
maturational-based theories (Bradley 2000). However correlation studies
are not conclusive for it is not known whether other variables account for the
observed behavior (Bradley 2000). In contrast, learning-based
theories consider the environment and infants’ interaction with it as the main
factor in shaping infant behavior (Bradley 2000) . The model, we propose
interacts with its environment (plans and executes grasp actions) and observes
the consequences of its actions (grasp feedback) and modify its internal
parameters (corresponding to neural connections) such that certain patterns
(grasp plans) are selected and refined amongst many other possibilities. Thus,
in this sense, our model conforms the learning-based views of motor
development.
Infants lack the ability to fully utilize their vision for
grasping during the period of early grasping (Newell et al. 1989;
Rosenbaum 1991).
Nevertheless, infants, as reviewed in the previous section, can grasp objects
and even adjusts their grasp actions according to object’s visual properties.
Thus, we propose that infant grasp learning is mediated by neural circuits
specialized for grasp planning, which can function with limited visual analysis
of the object, probably position and rough size. The macaque monkey was shown
to have a specialized circuit for grasping in which parietal areas extract
object affordances (information relevant for grasping) (Taira et al. 1990) and relay those to premotor
cortex. Then the premotor cortex with various contextual and intention related
bias signals performs grasp selection/execution task (see Fagg and Arbib 1998
for a modeling study).
It is very likely that a similar circuit exists in human (Jeannerod et al. 1995) which is adapted in infancy
to provide skillful grasping in adulthood.
We present Infant Learning to Grasp Model (ILGM) in two
stages. The first stage is the period when infants are unable to incorporate
object affordance into grasp plans while the second phase is when infants start
incorporating object information into grasps. Although we favored
learning-based theories of motor development, ILGM is compatible with both
maturational- and learning-based motor development schools since in either
case, with different reasons though, the affordance information cannot be used
by the grasp learning circuit in early infancy but it becomes available as
development progresses.
In the rest of the chapter, we analyze ILGM via simulation
experiments, and present behavioral responses and make comparisons where
experimental data is available. When no data is available, we produce useful predictions
that can be experimentally tested.
With the term learning to grasp we mean to learn how
to make motor plans in response to sensory stimuli such that the open loop
execution of a plan leads to a successful grasp. There is strong behavioral
evidence that early grasping is based on open-loop control and does not use
visual feedback (Clifton et al. 1993;
Clifton et al. 1994; von Hofsten and Ronnqvist 1988; Streri 1993). Chapter 7, studies the
visual feedback control of grasping in a simplified setting
We first describe a generic schema level architecture of
Learning to Grasp Model. Later sections use the general architecture introduced
here to conduct simulation experiments. We propose three computational layers
relevant for grasping: Hand Position, Virtual Finger, Wrist Rotation layers. In
general, the input to our network is the affordance of the target, which can
vary from a single quantity indicating the existence of a graspable object to
the full description of the object in terms of its affordances (e.g. size,
orientation, etc.). In later sections when running simulations, we engage
layers according to experimental requirements. For example to simulate early
infant grasp learning, we effectively disable Affordance layer and analyze
learning with tactile feedback. The affordances are represented using
population-coding scheme and encoded algorithmically (i.e. no visual processing
for object recognition and feature extraction is done). We use Affordance
layer or Input terms interchangeably to conform the context. The
layers we introduced here encode motor parameters that constitute a minimal set
for specifying grasp actions and based on behavioral studies and monkey neurophysiology.
We make use of monkey studies by postulating that
monkey and human motor development follows similar patterns In fact the
kinematics of reach to grasp movements of the macaque monkey and human is very
similar (Roy et al. 2000) and homologous brain
structures are involved in motor tasks (Jeannerod et al. 1995) .
Adult grasping studies suggests that reach and grasp
components of a grasping action is independently programmed (Jeannerod and Decety
1990).
This implies that the position of the fingers (hand configuration) on the
object determined first, and then based on the hand configuration wrist
orientations and arm configuration are determined. However in the infant,
reaching component dominates: infants first learn how to reach and then learn
to adjust their hands to match according to their ballistic reach (Clifton et al. 1993;
Clifton et al. 1994; von Hofsten and Ronnqvist 1988; Streri 1993). Therefore, we suggest that a
grasp planning inversion takes place after infants become skillful. The
inversion is required because intended manipulations after a grasping action
determines how humans grasp the objects. If one were planning to grab a tennis
ball with the aim of throwing it far, probably a power grasp would be
preferred. On the other hand, if he/she were going to pass it to a person next
to him, a (tripod) precision grasp would be more appropriate.
In accordance to infant development, we propose that infants
explore the space around the objects (and occasionally touch them) they
interact. Thus, infants’ early reaches can be considered object centered.
Early reaching is variable (see Bradley 2000 for a
review);
the variability must be reduced for successful grasping. We posit the Hand
Position layer as specifying the approach direction; that is the object
centered (allocentric) position of the hand from where it will approach the
target.
Given an approach direction, the orientation of the wrist
has to be determined. For example, when approaching a sphere from the bottom
side, a large wrist supination is required while approaching from the front a
wrist flexion would be required. We posit the Wrist Rotation layer to learn the
possible wrist orientations given the allocentric hand position information
relayed from Hand Position Layer. Wrist Rotation layer also receives
projections from the Affordance layer, because in general, different objects
afford different set of approach-direction and wrist-rotation pairs.
The Virtual Fingers layer indicates which finger synergies
will be activated given an input. This layer’s functionality is fully utilized
in adult grasping. When infants start learning to grasp, they first engage all
of their fingers in a synergistic way. When infants start to control their
digits independently to match object shape, this layer can be engaged in
learning the possible virtual finger activations. In the simulations that will
be presented in this chapter, the Virtual Fingers Layer is always used to
specify a synergistic control of the fingers but is included in the ILGM
description for completeness. As will be shown, this does not refrain us from
reproducing infant behavior and generating testable predictions.
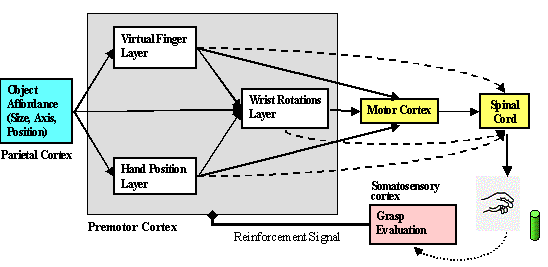
Figure 5.2 The structure of the Infant Learning to
Grasp Model. The individual layers are trained based on somatosensory feedback
The layers we proposed are in one-to-one correspondence with
Iberall and Arbib’s (1990) schemas for grasping. The
schemas of Preshape, Approach Vector and Orient correspond
to the ILGM layers of Virtual Fingers, Hand Position and Wrist
Rotation (Iberall and
Arbib 1990).
The layer architecture we introduced in Chapter 3 is
instantiated (as a population of
neurons) for the parameters, allocentric hand position, virtual fingers and
wrist rotations that determine a grasp plan. The key features of the
architecture introduced in Chapter 4 are that it is capable of representing
multiple choices of actions and it is open to biasing. The model, after
learning, will retain a menu of grasp actions that can be retrieved based on
the affordance. The menu then can be biased to satisfy task constraints. Figure 2.1 shows schematically the structure of the ILGM. The
task division of brain regions for implementing the schemas left to the next
chapter, in which we probe the possible brain localization of grasp learning
based on the monkey neurophysiology. For now, we only give an overall view of
grasping circuit. Parietal areas extract object affordances and relay them to
the premotor cortex, which is the center implicated in grasp programming. In
turn, premotor cortex makes a grasp plan and instructs the spinal cord and
motor cortex for execution. The result of the plan is integrated in the first
somatosensory cortex (SI). Output of somatosensory cortex mediates the
adaptation of the grasping circuit.
Hand Position layer determines where the hand will be
with respect to the object during grasping. Intuitively, this represents the
side from which the hand will grasp the object. The frame of reference for this
parameter is allocentric. The coordinate system we choose for this parameter is
spherical (see Figure 5.3). Our preliminary simulation showed that this choice
is advantageous over a rectangular coordinate system because the spherical
representation was less sensitive to errors in radius component while the
rectangular coordinate system had the same sensitivity for all the components.
The encoding used is as described in Chapter 4: the neural layer represents a
probability distribution and the values are read of by local population vector
computation. Given an object, the layer represents the feasible positions for
the hand. Any item from this position ‘menu’ can be selected by external
circuits. However, we simulate the grasping circuit autonomously, by processing
the input and generating a grasp program according to the probability
distributions represented in ILGM layers (but see Fagg and Arbib
1998 for prefrontal biases for grasp selection ).
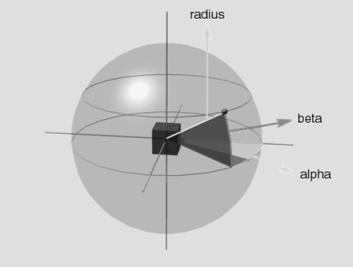
Figure 5.3 Hand
Position layer specifies the approach direction of the hand towards the object.
The representation is allocentric (centred on the object). Geometrically the
space around the object can be uniquely specified with the vector (azimuth,
elevation, radius). The Hand Position layer generates the vector by a local
population vector computation. The locus of the local neighbourhood is
determined by the probability distribution represented in the firing potential
of Hand Position layer neurons (see Chapter 4, for details)
Virtual Fingers layer specifies the fingers that will
be activated, and with what strength. We use three virtual fingers: thumb,
index finger, and the remaining three fingers acting together. The processing
is parallel to the Hand Position Layer’s flow. However, this does not mean that
this layer has the right to decide the virtual fingers on its own: the virtual
fingers that cannot yield grasping are negatively reinforced and hence do not
appear in the learned ILGM.
Wrist Rotation layer funnels all the information
about the object (affordance) and the Hand Position and Virtual Finger layers’
output. Thus, the output of this layer represents the possible wrist
orientations given the (1) object related input, (2) output of Hand Position
layer (3) output of Virtual Fingers layer in terms of a conditional probability
distribution. The parameters generated in this layer determine the movements of
wrist extension-flexion (tilt), wrist supination-pronation (bank) and ulnar and
radial deviation (heading).
Infants are almost preoccupied with manual manipulation.
Infants would play with their own hands; manipulate objects given to their
hands, and play with rattles before they can reach to grasp for them as young
as 2 months of age (Bayley 1936). Infants, once contacted the
object, occasionally would try to grasp it (Clifton et al. 1993). We suggest that the tactile
stimuli induced neural signals motivate infants to engage in grasping and
holding. However, we do not model the mechanisms of ‘joy of grasping’ induced
by tactile sensation but instead use a physical substitute to emulate the
feedback that infant would receive when grasping an object
Through ‘joy of grasping’ infants explore and learn actions
that lead to grasp-like experiences. What we call ‘joy of grasping’ can be
considered as Sporns and Edelman’s (1993) adaptive value of an
action. Sporns and Edelman’s (1993) postulate three concurrent
steps for sensorimotor learning: (1) The spontaneous movement generation (2)
development of the ability to sense the effects of movements, eventually
allowing neural selection to be guided by adaptive value (3) actual selection
of movements based on the adaptive value. Furthermore, it is argued that
selection in the nervous system is mediated mainly via synaptic change (Sporns and Edelman
1993; Sporns et al. 2000)
supporting our model’s relevance to infant learning.
The model we develop in the chapter will show how sensory
feedback shapes infant reaches into grasp actions via explorative learning and
produce testable predictions (see Sporns and Edelman
1993 for a simple reach learning architecture based on similar principles).
A successful grasp requires that the object stays stable in
the hand (must not drop or move) (MacKenzie and Iberall
1994),
which is physically defined as the following (Fearing 1986)
(1) The net force (Fnet)
acting on the object must be zero
 where Fi denotes the force applied by the
fingers at contact i and Ni
denotes the normal of the surface that is involved in the contact. M
denotes number of contact points of the hand on the object.
where Fi denotes the force applied by the
fingers at contact i and Ni
denotes the normal of the surface that is involved in the contact. M
denotes number of contact points of the hand on the object.
(2) The net torque (Tnet) acting on the object
must be zero
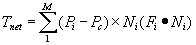 where Pi
is the contact position and Pc is the center of the mass of the object.
where Pi
is the contact position and Pc is the center of the mass of the object.
(3) For any force acting on the object, the angle between
the directions of the force with the surface normal must be less than a certain
angle f.
This angle is defined through the finger and contact surface dependent
coefficient m
with the relation:
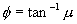
The constant m satisfies the property that
if Fn and Ft are the normal
and tangential components of an applied force then there will be no slip if
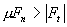
(4) The magnitude of the grasping force should be adaptable
to prevent any displacement due to an external force.
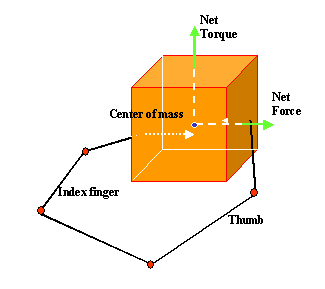
Figure 5.4:The grasp stability we used in the
simulations is illustrated for a hypothetical precision pinch grip (note that
this is a simplified, the actual hand used in the simulations has five fingers)
The parameters generated by Virtual Fingers layer determine
how much each virtual finger is activated. By converting these values to forces
exerted on the object during contact, ILGM, in theory, can discover the force
values that will stabilize the object. However, in our preliminary simulation
studies, we have seen that learning to grasp required excessive grasp trials to
discover the force balance.
As mentioned before we do not intend to model the details of the tactile
feedback system our aim is to compute a value that captures the joy (behavioral
reward) of the infant during grasping or a neural signal (neural level reward)
that indicates a stable grasp. Therefore, we concentrated on the question of
whether, given an object, the simulated hand’s contact configuration could
afford a stable grasp. We formulated the problem as a constrained minimization
problem with the cost function E.
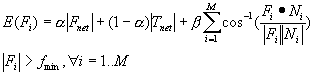
The values a and b determine
relative contributions of the individual costs terms to the total cost
function, E. The first two terms capture the grasp stability
conditions (1) and (2); the last term captures the grasp stability condition
(3).
The value fmin is an arbitrary positive constant to
avoid the degenerate solution (Fi=0).
To test the hypothesis that goal directed reaching could be
the basis of infants’ early grasp learning we mimicked the infants’ elemental
hand behaviors: we implemented palm orienting behavior and enclosure reflex in
the 3D hand/arm which we developed for Reach and Grasp schema of Chapter 3.
During the neonatal period when an infant reaches towards an
object, his/her hand is usually open (von Hofsten 1982). Although this behavior
disappears before the second month of age; it resumes around the third month of
postnatal age (see Bradley 2000). It has been suggested that
enclosure reflex constitutes the first stage of grasping (Twitchell 1970; see
Streri 1993 for a review).
In our model we wanted to test whether
reflex based grasps can be factored into grasp plans so that instead of
a randomly directed reach (before learning stage), the infant can use the
reinforced directions that yield appropriate contact with the object so that
enclosure of the hand yields a stable grasp.
The simulation is set up as the following. The model
generates a grasp plan. In this restricted case, the grasp is determined by the
(allocentric) hand position parameter which is a triplet describing a point
with respect to the target. The value is read off from the population activity
of the neurons in the Hand Position layer (see Chapter 4 for details of layer
operations). Initially, the value is random since we initialize the weights to
small random values. The Hand Position layer captures the infant’s variable reaches
towards the visual targets. The task of learning is then to narrow down the
variability to account for only appropriate approach directions (for a given
object at a certain location).
After a grasp plan (approach direction) is generated, the
hand starts executing the reach action specified by the Hand Position
parameter. During the reach, the wrist is rotated so that the palm normal
always points to the target object. From literature, we know that infants
(except a one month period) open their hand while they are reaching for objects
(von Hofsten 1982) and orient their palms
towards the them (see Streri 1993 pages
46-47).
As the model generates grasp plans and executes them, it
receives rewards from those plans that yield grasp or close-to-grasp actions.
In the implementation, the model receives negative reward for plans not
yielding stable grasps. However, when the grasp error, E is small (e.g.
the grasp is close to stable) the model receives a positive reward that is
proportional to e-E.
Thus, an almost-grasp program is encouraged which enables the model to have a
higher chance of producing similar plans. This makes our approach goal directed
and different from pure trial and error learning.
Figure 5.5, right panel, illustrates a discovered grasp (i.e.
approach direction) by the model. The model, by interacting with the object,
learned that it is possible to grasp the object from the bottom side. The grasp
plan our model generates is defined by a triplet (a,b,r) (a
stands for azimuth; b
for elevation and r for radius) as shown in Figure
5.3. If we sum over the radius we can represent the grasp
plan as a pair and plot it as shown in Figure
5.5, left panel. We can think of this plot as the
normalized histogram of (a,b)
pairs generated by the model over many trials. The plot tells us that the model
prefers grasp directions from a > 00 (below the
object) and b
<900 (right and/or front
of the object). The position of the object allowed the arm to grasp the object
from bottom and front/right. For example, a trial with an approach to the
object from the top does not yield a stable grasp (the length of the arm limits
the position of the hand. Those positions that could not be reached do not
yield a stable grasp when the hand contacts the object).
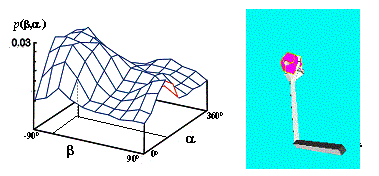
Figure 5.5 The trained model’s Hand
Position layer is shown as a 3D plot. One dimension is summed to reduce the 4D
map to a 3D map. Intuitively the map says: ‘when the object is above the
shoulder and in front grasp it from the bottom’
We
also tested the model when the object is located at a different place in the
workspace.
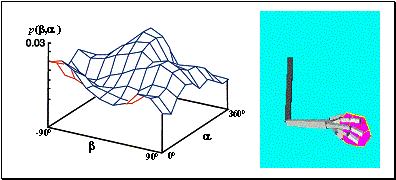
Figure 5.6: The
output of the trained model’s target position layer is shown as a 3D plot. One
dimension is summed to reduce the 4D map to a 3D map. The object is on the left
side of the (right handed) arm. Intuitively, the map says ‘when the object is
on the left side grasp it from the right side of the object’
This
time the object is placed rather low in the workspace on the left side of the
(right-handed) arm. Here we observe that the model discovered that a grasp
directed to the right hand side of the object is likely to result in a stable
grasp. Figure
5.6 shows the results of this simulation. Figure 5.7 shows the distribution of the Hand Position layer
during training. Initially the probability distribution of approach directions
was set randomly. The distribution gets a regular pattern via learning and the
reward yielding regions gain higher levels of activity.
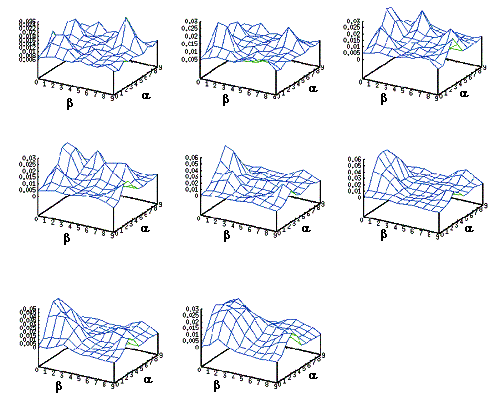
Figure 5.7 The
learning evolution of the distribution of the Hand Position layer is shown as a
3D plot. Note that the 1000 neurons shown represent the probability
distribution of approach directions. Initially, the layer is not trained and
responds in a random fashion to the given input. As the learning progresses,
the neurons gain specificity for this object location.
The simulation results showed that goal directed reaching
with palm orienting behavior is enough for generating power grasps. The model
predicts that infants will narrow their reach variability when presented with
objects that they can interact with. Furthermore, the variability reduction
will be more pronounced for the space where they could grasp objects.
In this section, we deprive the simulator from auto
palm-orienting behavior to test whether the infant palm-orienting behavior can
be mediated via learning, rather than being innate. Although there are accounts
that infants usually orient their hands during reaching so to increase the
likeliness of a contact between palm and the object (von Hofsten 1982), to our knowledge, there is
no account of innateness of this behavior. The period we are modeling
corresponds to early grasping in development when the object affordance is not
available to the grasping circuit, either because of maturational shortcomings
or because the complexity of learning holds back the motor development (in
accordance to maturational- and learning-based theories of motor development,
respectively).
In terms of learning task, ILGM has to discover the
distribution of wrist movements (supination-pronation, extension-flexion,
ulnar/radial deviation) for multiple approach directions. Presented with the
object, Hand Position layer produces the distribution of possible approach
directions. The selection is relayed to Wrist Rotation layer. Then, Wrist
Rotation layer computes the distribution of feasible wrist orientations
conditioned on the generated hand position. Virtual Finger layer generates
synergistic parameters effectively learning a single parameter to dictate the
enclosure speed of the hand as a whole.
The model learned to generate parameters to perform power
and precision grasps, and many variations of the two. The most abundant grip
generated was the power grip and its variations. Precision type grasps were
less frequently generated.
In earlier studies, it was thought that infants were unable
to demonstrate precision grips during grasping (Halverson 1931). The newer studies showed
that, when infants are tested in proper conditions, perform precision grips
occasionally (Butterworth et al.
1997; Newell et al. 1989).
ILGM simulation results are in accordance with this finding. The fact that
power grasp is inherently easier manifests itself in both infants and ILGM
simulations: As long as the object is brought in contact with the palm, an
enclosure (as in palmar reflex) is likely to produce power grasp. Figure 5.8, shows the grasping of a cube with a power grasp plan
generated by learned ILGM.
Figure 5.8 ILGM
planned and performed a power grasp after learning. Note the supination (and to
a lesser extent extension) of the wrist required to grasp the object from the
bottom side
The learned precision grasp varieties were mainly involved
the engagement of fingers other than thumb and index finger. Figure 5.9 shows two examples for this. Usually the object is
secured between three or four fingers, thumb opposing the center of remaining fingers.
This emergent grasping behavior is in accordance with the theory of virtual
fingers and opposition spaces (Iberall and Arbib 1990) and human tripod grasping (Baud-Bovy and Soechting
2001).
Figure 5.9 Two
learned precision grips (left: three fingered; right four fingered) are shown.
Note that the wrist configuration for each case. ILGM learned to combine the
wrist location with the correct wrist rotations to secure the object
Figure 5.10 show examples of two finger precision grasps which
were less frequently generated than the three or four fingered precision
grasps. These results show that even without object affordance, precision type
grips can emerge from a circuit adapted using supervised Hebbian learning,
which can be employed by infants.

Figure 5.10 ILGM
was able to generate two fingered precision grips. However these were less than
the three or four finger grips
The result shows that even without object affordance, performing
reaches directed towards the object from various allocentric positions and
associating the grasp-yielding wrist orientations with allocentric positions,
and a variety of grips, including the precision grip, can be learned predicting
the results of the study of Butterworth et al. (1997). Thus, infant can learn to
select the ‘right’ grasp from the grasp ‘menu’ based on internal motives or
environmental constraints. There are two observations that have to be made:
Firstly, the secondary learning mentioned here is easier to master. Infants
only have to associate the object properties (and the context) to the correct
configuration that they have already achieved. Secondly, no extrinsic to
intrinsic transformation is required because a posture that yields a successful
grasp has already been discovered. In computational terms, supervised learning
can take place.
The normative developmental phases of infant prehension
starts with palmar reflex, followed by power grips; and finally, ends with
dexterous finger-thumb opposition precision grips (Halverson 1931; see
Newell et al. 1989 for a review). However, this early view has been challenged by
advanced recording techniques and careful experimental conditions (Newell et al. 1989;
Butterworth et al. 1997).
If a variable (e.g. infant grip configuration) is dynamically context specific,
the experimental approach may be too artificial to reveal its effect on motor
control (Bradley 2000). For example, the postural
requirements could have been a factor in masking infant grasp abilities in the
earlier infant motor development studies (Newell et al. 1989;
Bradley 2002).
Task constraints may be viewed as including the goal of the task or the rules
that constrain the response dynamics (Newell 1986). Some examples of task
constraints are the object properties such as size and shape (Newell et al. 1989). To verify the hypothesis
that infant grasping is shaped by task constraints we designed a simulation experiment
with a physical constraint as the following.
In the earlier simulations of ILGM we presented the object
without constrains, hanging in space, thus the model could grasp the object
from all anatomically possible directions. However, this is not usually the
case for the infant. For example, infants often interact with objects that lay
on flat surfaces such as the floor or table. A small object on a horizontal
table is grasped best by opposing index finger and thumb with an approach from
top.

Figure 5.11 The cube
on the table simulation set up. ILGM interacts with the object with the
physical constraint that it has to avoid collision with the table
We simulated the situation by presenting ILGM with a small
cube placed on a horizontal plane (Figure
5.11). The plane constituted a physical obstacle for many
grasp attempts. Thus, when the simulated hand/arm collided with the table, a
negative reward was returned to ILGM. We let ILGM interact with the cube on
the table and analyzed the acquired grasping behavior.
ILGM with the cube on the table task condition was
unable to acquire power grasps (whole hand prehension). The grasp attempts of
the model that would result in a whole hand prehension resulted in negative
rewards as the fingers always collided with the table surface. Thus, the grasp
plan parameters yielding power grasps were not represented in the grasp
repertoire after learning.
The approach directions for avoiding the collision were
learned perfectly. LGM always attempted to grasp the cube from top. Figure 5.12 shows typical precision grips executed by ILGM after
learning. The grasp ‘menu’ acquired was composed of grasps with wrist positions
above the object. The contact points on the cube showed variability (see Figure 5.12).

Figure 5.12 ILGM
learned a ‘menu’ of precision grips with the common property that the wrist was
placed well above the object. The orientation of the hand and the contact
points on the object showed some variability. Two example precision grips are
shown in the figure
Many
of the precision grips that were learned involved supportive fingers other than
the thumb and the index finger. However, two finger precision grips were also
acquired (see Figure 5.13).

Figure 5.13. ILGM
acquired thumb opposing index finger precision grips
Most of the precision grips learned correspond to inferior
forefinger grasp (Figure 5.12) and inferior pincer grasp (Figure 5.13) , according to classification of Butterworth et al.
(1997) (see Figure 5.1). One of the interesting observations is that ILGM
assimilated the object affordances into the grasp ‘menu’ it learned. By
comparing Figure 5.12 and Figure
5.13, we see that the opposition axes used for grasping
were 90 degrees apart. In both grasps of Figure
5.12, the thumb was placed on the left surface of the cube
whereas in Figure 5.13, it was placed on the surface that is parallel and
closer to the presumed infant.
Clearly, it is not possible to grasp a small object with the
whole-hand grip without further manipulating (e.g. dropping or raking) the
object or without deforming the hand to a pincher grasp (readers are encouraged
to grasp a pellet from a table without using precision grip). Infants, during
early grasping phase (4-6 months of age), certainly contact the surface before
they can grasp the objects as they use tactile senses for grasping (Newell et al. 1989). However, we predict that
infants’ ability to grasp small objects from a hard, flat surface using
whole-hand precision would be a rare, if not impossible, occasion. On the other
hand it has been shown that infants are able to use various precision grips (Newell et al. 1989;
Butterworth et al. 1997; Corbetta et al. 2000) which is in full accordance
with our findings. It appears that older infants are more interested in small
objects compared to younger infants. ILGM explains why, as infants grow older,
prefer to approach objects from top (Fagard 2000) with the answer that from-top
approach is the most natural way to grasp small objects in a constrained
condition like the one we presented (i.e. small object on the hard flat
surface).
The simulation results of cube on the table task
(combined with earlier simulation results) have nontrivial consequences. To be
precise, ILGM simulation results:
- Predict that infant even without object affordance
input, that is during the age of early grasping, can perform precision
grasp
·
Show that task constrains shape motor development and
support the view that development of precision grips is mediated by task
constraints.
·
Show that object affordances could be represented in
infants’ grasp repertoire in spite the fact that they are unable to access/extract
object affordance information. This result is very important because it is a
proof that tactile learning can train/modulate the visuomotor learning in
infancy. A corollary prediction is that infants learn to extract affordance of
the objects they can grasp.
·
Predict (in relation to the preceding item) that object
(visual) affordances would be heavily represented in the motor and sensory
areas of human cerebral cortex for objects that we manipulate often. For
example, presented with a drill, carpenter’s neural circuits would extract more
elaborate affordances than a fisherman’s would.
Until now, we simulated infant grasp learning assuming that
vision of the object is not used to adjust grasp plans according to object features.
Therefore, we can associate the earlier ILGM infancy period of two months to
six months of age. In this section, we introduce affordance input to ILGM and
associate the model with infancy period of nine months and after.
This section is reserved for simulating the study of Lockman
et al. (1984) and compare ILGM results with
their infant data. Lockman et al. (1984) used infants of 5 and 9
months of age and compared their performance in orientating their hands to a
dowel presented in horizontal and vertical position (see 5.2
section for more details). To replicate their experimental condition, we let
the affordance of the object (orientation) to be relayed to ILGM preferentially
for 5 and 9 months of age. We presented a cylinder to ILGM analogous to a
dowel. With the (realistic –see section 5.2) assumption that information about the axis
orientation (i.e. affordance of the object) is not available to the grasp
planning circuit during early infancy (5 months of age) and that it becomes
available later in development (9 months of age), we effectively disabled the
orientation encoding
when simulating the younger infants’ grasp learning whereas when simulating
older infants’ learning we enabled the orientation coding in the Affordance
layer. We refer to the former case as the poor-vision and the latter as
the full-vision condition.
We predicted that the infants without affordance input would
not be able to factor the object orientation into their motor plans. To make
the experiment a little bit more interesting, we also included a third,
diagonal orientation condition in addition to the existing horizontal and
vertical orientation conditions.
Lockman et al. (1984) used orientation difference
between the hand and the dowel as a measure of how much infants adapted their
hand orientations to the target. The experimenters scored the difference
between the hand orientation of infants and the dowel. Lockman et al. (1984) used 0 for full match and 4
for maximal mismatch (i.e. the difference between the orientations of the hand
and the dowel was closer to 90 than 67.5 degrees). Each of the sixteen infants
performed eight grasps totaling 128 grasps. The grasp definition used by
Lockman et al. (1984) however, was relaxed: a
finger wrapping the dowel would be counted as a grasp. In our simulation, when
ILGM was learning we used our grasp stability measure; when data collecting for
the analysis, to be compatible with Lockman et al. (1984), we included cases where the
grasp stability was not achieved.
Figure 5.14 The three cylinder
orientations and grasp attempts by the poor vision condition.
Figure 5.14,
centre grasp does not satisfy our grasp stability criterion but conforms to the
definition of Lockman et al. (1984).The right two grasps do not
satisfy the grasp stability criterion
Figure 5.14
shows the orientations and the cylinder we used in the simulation. The grasp
actions shown were performed by ILGM in the poor-vision condition. With our
grasp stability measure, we observed that in 10 trials the horizontal cylinder
could be grasped six times, The vertical cylinder could be grasped four times
and the diagonal cylinder could not be grasped at all. Using the convention of
Lockman et al. (1984), we saw that the horizontal
cylinder could be grasped eight times, the vertical cylinder five times and the
diagonal cylinder seven times. The numbers indicate that without affordance
input (orientation) the grasp learning was not satisfactory.
We first compare the averaged data from Lockman et al. (1984) and data from our simulator
over multiple runs (128). We compare the performance of the infants and the
simulator for the vertical oriented
cylinder (see Figure 5.15, right panel). Figure
5.15, left panel shows average orientation match score
versus reach progression for infants and for the simulation. The diamonds over
dashed line indicates the 5 months old infants’ performance. The 9 months old
infants adjusted their hands better than the younger ones as indicated with
diamonds over solid line.
Figure 5.15 The orientation match of the
hand and the cylinder is illustrated. Dashed line with diamonds: 5 months old
infants; Solid line with diamonds: 9 months old infants; Dashed line with
circles: ILGM with no affordance; Solid line with circles: ILGM with affordance
(infant data from Lockman et al. (1984)). Right
panel illustrates the object orientation used for the simulation and for the
infants in this comparison
The simulated ILGM data is shown with the same line style,
but data points are marked with circles. Although the absolute scores differ
between simulation and infant case, the performance improved in the full-vision
case similar to the performance improvement for 9 months old infants. Moreover,
the performance increment of ILGM and Lockman et al.’s (1984) infants were comparable.
Now we try to infer what the model learned. When we analyzed
individual errors made by the simulator, we observed six typical error curves (Figure 5.16). We grouped the error curves into rows to
differentiate the mode of operation learned. The columns, from left to right,
correspond to horizontal cylinder, diagonal cylinder and vertical cylinder.
Note that top-left graph is flat and shows almost zero error whereas the other
two graphs in the same row show flat curves with higher error.
Figure 5.16 The
hand orientation and cylinder orientation difference curves for individual
trials. The columns from left to right correspond to horizontal, diagonal and vertical
orientations. The upper row flat class of error curves, lower row non-flat
class for error curves (see text for explanation)
Lockman et al. (1984) found that infants start
their reaches with horizontal orientation so that their initial errors for the
horizontal dowel are low. Based on this finding the initial configuration of
the hand was set to a horizontal posture in our simulations. In accordance with
Lockman et al. (1984) observations, ILGM made more
corrections for the vertical cylinder case (bottom-right panel in Figure 5.16) and less for the horizontal case. From this we can
infer that in the upper row trials of Figure
5.16, ILGM model used a grasp plan appropriate for
horizontal orientation. As can be seen from the bottom-right panel, the model
can occasionally perform a vertical cylinder adaptation too (remember that the
architecture of ILGM allows representation of multiple grasp plans). However,
the model cannot differentiate the two grasping strategies. Thus, the model
learns a strategy to increase its chance to make successful grasps. Since the
initial hand posture is close to horizontal, it is intuitive that the dominant
mode of grasp planning becomes the one best suited for horizontal cylinder, as
it requires less correction (Lockman et al. 1984). The model replicates this
observation as the horizontal cylinder could be grasped most frequently.
ILGM in the
full-vision condition learns to perform grasps similar to 9 months of age
infants. We present the plots of difference of hand orientation and cylinder
orientations in Figure 5.17
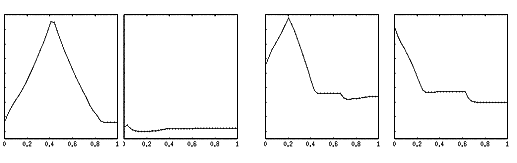
Figure 5.17 The
hand orientation and cylinder orientation difference curves while ILGM was
executing four types of grasp in the full-vision condition. Left two figures
are two typical error curves for the horizontal cylinder. Note that the two
horizontal case error patterns reflect the two possible grasps: from the bottom
and from the top. The third and fourth are typical error curves for the
diagonal and vertical cylinders respectively
The left two panels
show the difference in orientations for the horizontal cylinder case. The flat
curve corresponds to the easy grasp that could be observed in
poor-vision condition also. The other high curvature one corresponds to a
bottom grasp of the cylinder as shown in the left panel of Figure 5.18. To our knowledge, there is not report of bottom
whole hand grasping during infancy. (Lockman et al. (1984) also did not observe this kind of grasp). This
may be due to the general experimental set up; or the inability of infants to
work against gravity. In our simulation, we did not address the dynamics of the
arm. Therefore, both grasp actions are equal in their reward value. One could
incorporate a penalty term for energy use in the ILGM reward computation (which
evaluates how well the a grasp action has been). This would tilt the balance of
grasp choice towards the easy grasp. It would be interesting to find out
the developmental course of this particular grasp and the underlying reasons
why it does not appear early in development. One explanation could be that
gravity helps infants to contact with the object earlier as infants
engages in arm movements and fisted swipes in the presence of visible objects (von Hofsten 1984). Thus, the infant has more
experience approaching objects from the top and front. When approaching from
the bottom the gravity works against the infant since the infant has to
counteract gravity to grasp the object. One notable property of this bottom
grasp is that it did not appear when we simulated ILGM for replicating 5 months
of age infant behaviour; because ILGM had to find a strategy that will work for
three orientations and hence had no exploration potential to discover
alternative grasps. Fagard (2000) found that hand orientation
at object contact changed with age. Horizontal orientation (the easy
grasp) decreased and vertical ones increased from 5 months old infants to 12
months old infants.

Figure 5.18 The
grasps performed after ILGM learned the association between the wrist rotations
and the object affordance (orientation)
This chapter presented the Infant Learning to Grasp Model
(ILGM) and presented simulations yielding nontrivial predictions. We first
showed that even having a very limited set of behaviors and visual input,
explorative learning could yield grasping behavior and reduce the variability
of grasping actions. Then we studied the palm orienting behavior and test
whether it could be acquired through interactive learning. The results of this
study showed that object affordance was not a prerequisite for learning the set
of correct wrist orientations. ILGM learned a ‘grasp menu’ including precision
grips, which could be retrieved based on the approach direction.
In our simulations, the side grasp was not discovered.
Although infants do not exhibit side grasps (opposition of thumb to side of
index finger), we would like to point out the possible reason. The neural
architecture we introduced in Chapter 4 is capable of learning any reward
yielding grasp plan. However, the physics modeling of the arm and the objects
is not realistic. We did not model contact forces. Therefore, thumb opposing
the side of the finger cannot be counteracted by the side of the finger. Except
for the palm, the forces can only be exerted in the directions determined by
joint angles of each finger.
It should be emphasized that, the point of our modeling was
not to give a realistic rigid dynamics model of the arm/hand but rather propose
a grasping architecture that can learn to program grasps as long as a correct
reward signal is given by the environment. The learning we demonstrated, and
the variety of grasps we could generate met our aim.
During development, infants have to deal with constraints
and find ways to act within the limitations of the environment and the context.
We investigated the possibility that task constraints may play a role in
shaping infant’s grasping behavior. We simulated a situation where a small cube
was placed on a table. The model was asked to interact with this simple
environment. The grasping configurations learned by the model reflected the
task constraints. The model could not acquire any whole hand prehension grasps
but acquired grasps that reach the cube from the top avoiding a collision with
the table.
Finally we analyzed what affordance may add to ILGM
by simulating the experimental set up of Lockman et al. (1984). With this simulation we
showed not only the improvement in grasp execution (measured as the orientation
match between infant’s hand and the target dowel) but also the similarity of
the improvement pattern was comparable to Lockman et al.’s (1984) results, which indicates that
ILGM captured the behaviour of 5 and 9 months age infants via differential
affordance access.
Combining the summarized simulations we explicitly state
that ILGM:
·
Predicts that infants, even without object affordance
input (i.e. during the age of early grasping), can perform precision grasp
·
Shows that task constrains shape motor development and
supports the view that development of precision grip is mediated by task
constraints.
·
Shows that object affordances could be represented in
infants’ grasp repertoire in spite the fact that infants’ are unable to
access/extract object affordance information.
·
Predict that the distribution of object (visual)
affordance in motor and sensory areas in human cerebral cortex would reflect
the frequency of their manipulation.
The last two results are very important because it shows
that tactile learning can train and modulate the visuomotor learning during
infancy. By projecting this statement back to infants, we predict that infants
learn to extract visual affordances of the objects they interact with.
The simulations with ILGM explored both the early grasping
period when infants are unable to factor object affordance into grasp plans and
the period when they start using visual input for grasp planning. We captured
the infancy period by limiting the visual input available to the model.
From a maturational-based theory point of view, ILGM should
correlate more with a phylogenetically older grasping circuit with limited
access to visual areas. One candidate for such a circuit is the primary motor
cortex that receives object location information from the superior colliculus.
As the cortical control becomes dominant while the infant is growing, motor
cortex leaves the grasp-planning task to higher cortical areas, probably to
premotor cortex, in adulthood.
From viewpoint of learning-based theory motor development,
infant and adult grasping circuits are identical but infants have to sort out
the flux of information they receive and organize them into useable schemas by
interactive learning. In contrast to maturational-based theories,
learning-based theories suggest that development of motor abilities is the
consequence of learning by trial and error to control motor schemas that are
genetically determined in their rudimentary forms (Bradley 2000). Thus, grasp planning and
execution mechanism can manifest itself only after the infant has interacted
enough with the environment. This is the period required for infant to master
his/her sensorimotor skills, which starts from birth and continues until the
first year of life. The movements of newborns are usually treated as
unintentional, purposeless or reflexive muscle activities (van der Meer et al.
1995),
probably with a bias from maturational-based theories. However, there exists
strong evidence that infants engage in learning and exploring actively as early
as 10 days of age. van der Meer et al. (1995)
recorded spontaneous arm-waving
movements of newborns while they were allowed to see only the arm they were
facing, only the opposite arm on a video monitor, or neither arm. The newborns’
hands were pushed downward in the direction of the toes. When the newborns
could see their arms, either directly or via the video screen, they opposed the
forces and moved normally, effectively preferring to have feedback on their
movements. The findings indicate that newborns control their arm movements as long
as they can receive visual feedback even when they have to oppose external
forces. This shows that babies at a very early age start exploring and
collecting data indicating that their visuomotor learning hardware is
functioning soon after birth.
In Chapter 5, we presented the schema level model of grasp
learning (ILGM) without spelling out the brain regions that contribute to
learning. In this chapter, we constrain ILGM with neurophysiological and
neuroanatomical data to pin down the brain regions involved in grasp learning.
Our analysis leads to two alternative hypothesis of primate grasp circuit. We
present evidence for both hypothesis and analyze the one that is best explained
by experimental data through simulation. In particular, we propose the
Affordance-based Grasp Learning model (LGM) which meets the neurophysiological
and neuroanatomical constraints and functional requirements derived from ILGM.
Thus, ILGM is functionally equivalent to LGM. We will refer the reader to
Chapter 5, when the material to be presented is already introduced there unless
it is necessary to have an overlap to keep the Chapter self-contained.
Our analysis of grasp learning in terms of neurophysiology
complements the Mirror Neuron System model we presented in Chapter 3. The
simulation results enable us to explain the mechanism of grasp learning in
terms of brain circuits and show how adaptation shapes the visuomotor
transformation that enables primates to select and execute suitable grasps
based on the object affordances leading to units with properties similar to F5
canonical neurons. Through simulation experiments, we make explicit
predictions, which can be tested experimentally and used for refining and
validating (or invalidating) the model we propose.
One of the basic assumptions of the Mirror Neuron System
(MNS) model (Chapter 3) was that self-observation of grasping was the training
stimuli adapting parietal and premotor circuits of MNS model for action
recognition. Using schema methodology (Arbib et al. 1998), the grasp learning and
execution were encapsulated under the Reach and Grasp Schema, which included a
set of algorithmic routines implementing reach and grasp execution based on
techniques from robotics. Learning to Grasp Model (LGM) of this chapter serves
also, as the biologically realistic schema, substituting the engineered Reach
and Grasp Schema of MNS. Figure 6.1
highlights the relevant regions for grasp learning using the schema level view
of MNS that we have presented in Chapter 3.
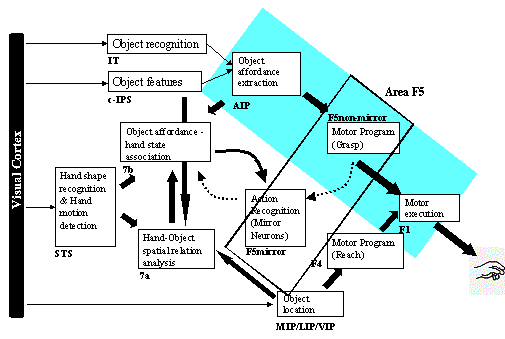
Figure 6.1: The
overall MNS model. The grey background rectangle shows the focus of this
chapter. In addition to the areas shown, area F2 will be posited as being
involved in grasp planning.
Many possible grasps can
be applied to objects and they require many control parameters to be adjusted
based on the object and hand properties. Iberall and Arbib (1990) introduced the theory of virtual fingers and opposition space for
reducing the complexity of the grasping task. The term virtual finger
is used to describe the physical entity (one or more fingers, the palm of the
hand, etc.) that is used in applying force and thus includes specification of
the region to be brought in contact with the object (‘virtual fingertip’). Figure 6.1 shows three types of opposition: those for the
precision grasp, power grasp, and side grasp. Each of the grasp types is
defined by specifying two virtual fingers, VF1 and VF2, and the regions on VF1
and VF2 which are to be brought into contact with the object to grasp it. Note
that the "virtual fingertip" for VF1 in palm opposition is the
surface of the palm, while that for VF2 in side opposition is the side of the
index finger. The grasp defines two "opposition axes": the opposition axis in the hand joining the
virtual finger regions to be opposed to each other, and the opposition axis in the object joining
the regions where the virtual fingers contact the object. Visual perception
provides affordances (different ways
to grasp the object); once an affordance is selected, an appropriate opposition
axis in the object can be determined. The task of motor control is to preshape
the hand to form an opposition axis appropriate to the chosen affordance, and
to so move the arm as to transport the hand to bring the hand and object axes
into alignment.
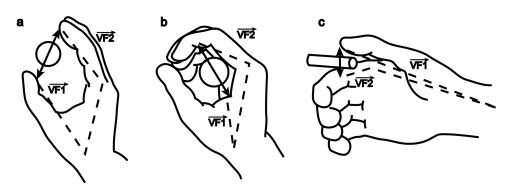
Figure 6.2 Left: precision grasp (pad opposition);
Middle: Power grasp (palm opposition); Right: Side grasp (side opposition).
Each of the 3 grasp types here is defined by specifying two ‘virtual fingers’,
VF1 and VF2, which are groups of fingers or a part of the hand such as the palm
which are brought to bear on either side of an object to grasp it. The
specification of the virtual fingers includes specification of the region on
each virtual finger to be brought in contact with the object. A successful
grasp involves the alignment of two "opposition axes": the opposition axis in the hand joining the
virtual finger regions to be opposed to each other, and the opposition axis in the object joining the
regions where the virtual fingers contact the object (adapted from Iberall and
Arbib 1990)
From a robotics viewpoint, we can model the grasping process
by extending the techniques available for trajectory planning by formulating
the grasp planning as an inverse kinematics problem. Indeed, we used this
approach when we studied the Mirror Neuron System Model to generate grasp
actions for providing visual input stimuli for the MNS model (Chapter 3).
However, in this chapter, we take a learning approach and propose a model that
learns to generate successful grasp plans via exploration and selection. By
doing so we aim at satisfying these goals:
1.
Shed light on the possible organization of the primate
premotor circuit involved in grasp planning by offering biologically realistic
learning rules and structures.
2.
Complement our earlier work on the Mirror Neuron System, by
substituting the engineered grasp-planning module of Chapter 3 with
biologically realistic and self-organized grasp-planning circuit
3.
Form a solid basis for hypotheses about Mirror Neuron System
development and visuomotor learning in parietal and premotor circuits, which
can be experimentally tested and further investigated with modeling studies
following the structure we propose.
The corticospinal tract is one of the main neural substrate
for independent finger control (Triggs et al. 1998). Firstly, corticospinal
projections terminating in the ventral horn innervating hand muscles predict
independent finger use for small objects (Bortoff and Strick
1993).
Secondly, lesioning the corticospinal tract prevents the development of the
independent finger movement in infant animals (Lawrence and Hopkins
1976),
and impairs independent finger movements. Human patients recovering from
corticospinal lesions initially tend to perform synergistic movement of all the
fingers as in a power grip (Denny-Brown 1950;
Lassek 1954).
More evidence is presented by Olivier et al. (1997). Hinde and Rowell (1964) observed that dexterous
grooming was not observed in infant macaques until 6 months of age. Lawrence
and Hopkins (1976) reported that in rhesus
monkey, the earliest signs of skillful hand use appear around 2-3 months and
stabilizes as a mature pattern at 7-8 months. Galea and Darian-Smith (1995) reported that performance on
a reach-and-grasp test (with infant macaques) reached adult levels around 6
months. More importantly, this correlated with the emergence of an adult-like
distribution of cortical motor areas contributing to the corticospinal tract.
Armand et al. (1997) showed that at birth CM
projections from primary motor cortex are very weak or not present. It is not
very clear how much information these weak projections may carry at an early
age. However Flament et al. (1992) reported that the earliest
EMG responses to transmagnetic stimulation as in the adult could not be
obtained before 2-3 months of age.
The division of function between premotor and motor cortex
projecting to corticospinal tract is not well studied. The primary motor cortex
has circuitry to facilitate grasping once a proper contact with the object is
established (Rothwell 1994). Certain primary motor cortex
neurons that control the finger muscles have cutaneous receptive fields on the
skin that likely encounter obstacles and may be stimulated when a movement is
caused by the neurons’ activity (Rothwell 1994). Similarly, the joint
receptors, tendon organs and muscle spindles have afferent organization such
that if the microstimulation of a certain primary motor cortex patch produces a
movement in one direction then the passive movement of the same joint in
the opposite direction is likely to excite that area of the cortex (Rothwell 1994). This means that, at least
for spindles, if a muscle is passively stretched, the afferents of the spindles
will activate primary motor cortex neurons, which would in turn produce
contraction of the same muscle (Rothwell 1994). Thus, the intrinsic wiring
of the primary motor cortex can enable grasping and holding of the object once
it is touched. Fogassi et al. (2001) showed with reversible
inactivation studies that the (precise) grasping behavior is compromised when a
muscimol injection was made to a certain part of area F5. Area F5 is divided
into two main sectors based on cytoarchitectonics (Rizzolatti et al. 1988): the F5 sector lying on the
cortical convexity (F5 convexity) and the part buried in the arcuate sulcus (F5
bank). Both sectors have neurons that respond to visual stimuli. The visual
neurons of F5 bank respond to the presentation of three-dimensional objects,
usually, in a congruent way with their motor responses in terms of grip type
and size (Rizzolatti et al. 1988;
Murata et al. 1997b).
The visual neurons in F5 convexity fire when the monkey observes an individual
performing certain actions involving object interaction (Gallese et al. 1996;
Rizzolatti et al. 1996a).
The former neurons are named ‘canonical neurons’, whereas the latter ones are
named ‘mirror neurons’. Although the muscimol injection to the region of F5
mirror neurons did not impair grasping behavior (only a slowing down was
observed), an injection made to F5 bank impaired (precise) grasping (Rizzolatti et al.
1996a).
The hand was not adjusted according to the object size and shape. Nevertheless,
the monkeys could perform the grasp after they touched the object.
Interestingly, the grasp for large objects appeared almost unaffected (Murata et al. 1997b). In one of the monkeys
tested, the use of the contralateral hand was largely impaired with F5 bank
injection. This monkey often refused to make reaching-to-grasp movements toward
small objects and, when it made them, grasping was clumsy and the hand shape
was inappropriate for the object size and shape. A large sphere and a large
cylinder, however, were grasped almost normally (Fogassi et al. 2001). The other monkey had similar
deficits but to a lesser extent. In both monkeys, the ipsilateral hand
performance was also compromised (when the muscimol injection was strong). It
is important to note that, finger dexterity was not abolished when F5 bank was
inactivated because after contact with the object the grasps could be
completed. This is very similar to the description of grasp performance during
early grasping phase of human infancy (von Hofsten and
Ronnqvist 1988).
Fogassi et al. (2001) also injected muscimol in the
hand region of motor cortex (F1). The result was a strong impairment in the
capacity of the grasping of the hand contralateral to the injection site.
Unlike F5 injection, the ipsilateral hand was not impaired (Fogassi et al. 2001). Both monkeys with F1
injection, could reach the tray that holds the object, but with a stereotypical
flat hand configuration after which they retrieved (i.e. used their hands like
a rake) rather than grasped the objects. The hand section of area F1 receives
rich projections from area F5 (Matelli 1986). We can argue that F5
modulates F1 hand related neurons so as to engage them in a precision grasp in
the following way. We know that neither area F1 nor area F5 alone is enough to
perform a precision grip (Fogassi et al. 2001) and area F5 neurons become
preferentially active when the animal performs grasping actions with varying
degrees of grip specificity (Rizzolatti et al. 1988;
Murata et al. 1997b; Rizzolatti et al. 2000). Thus, we suggest that area
F1 needs to be modulated by area F5 in order to carry on the dexterous grasp
actions. This proposal is also supported by human imaging studies. Ehrsson et
al. (2000) showed, using fMRI, that when
the human subjects performed power grasp, the primary motor cortex of the
contralateral hemisphere showed increased activity whereas when the subjects
performed precision grasp both hemispheres were activated. Importantly, the
dominant activity was observed in the (ipsilateral) ventral premotor cortex,
which is the homologue of monkey F5 (Gallese et al. 1996;
Rizzolatti and Arbib 1998).
Ehrsson et al. (2000) suggested that the control of
fingertip actions with a precision grasp is mediated with neural circuits that
are different than the circuits involved in power grasp, which are
phylogenetically older. Further support for this proposal comes from the
studies of Muir and Lemon (1983). Some primary motor cortex
cells that project to motoneurons that mobilize hand muscles are active during
precision grasp execution but not for a power grasp execution, albeit the same
muscles can be mobilized in both grasps. This indicates that there exists a
skilled grasping circuit, which uses some part of primary cortex for its
exclusive function. Then, how much visual information is available to
phylogenetically older (primary motor cortex) grasping circuit and how well can
it learn sensory motor associations (as opposed to the fixed motor plans)? Shen
and Alexander (1997a) showed that the neurons in motor cortex
participate in sensory and/or associative (context-dependent) processing of spatial
information relevant to visually guided reaching movements. They have found
many neurons in motor cortex that showed behavior-correlated discharge that
depended on the visuospatial target of the monkey’s instructed reach,
irrespective of the limb trajectory used (as expected, there were also a
substantial proportion of neurons with limb-dependent activity as well).
This means that the primary motor cortex neurons indeed can encode motor plans
based on visual cues that are not tied to certain muscle groups.
We now present two alternative hypotheses of primate grasp
development that will guide us in locating the specific brain areas involved in
grasp learning in the following sections.
The first hypothesis is that the early power-like grasp is
mainly controlled by area F1 and F4 (for the reach component), and involvement
of area F5 is not substantial. The review in the previous section suggests the
existence of a phylogenetically older lower level grasp circuit that does not
require premotor regions and can work with limited visual analysis of the
object, probably position and rough size. The power grasp appears early in
development but it takes longer to mature a precision type of grip (Lockman et al. 1984;
Fagard 2000).
In addition, infants lack the ability to fully utilize vision for grasping
before the precision grip becomes part of their grasp repertoire (Rosenbaum 1991). Ehrsson et al. (2000) suggested that the control of
fingertip actions with a precision grasp is mediated with different neural
circuits than the phylogenetically older circuits for power grasp.
Hypothesis I explains the emergence of skillful finger use
as the following. The visual control of dexterous finger use (e.g. precision
grip) is learned by area F5 by associating the performance of the lower level
grasp circuit with the visual analysis of the object performed by parietal
cortex (AIP). Area F5 modulates grasp selection and the activity in the lower
level grasp circuit. The modulation and selection are based on the high level
goals of the individual (prefrontal influences based on context), affordances
extracted by parietal cortex, the lower level grasp plans and the actual
grasping performance (success or failure).
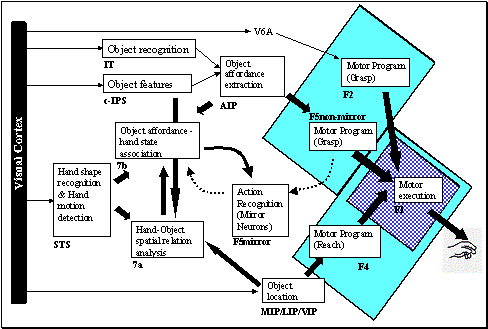
Figure 6.3 The two
possible organization of learning to grasp circuit are shown. According to
Hypothesis I, two grasping circuits exist; the phylogenetically older one
located in area F1 (hatched background) and the newer one in the premotor
cortex (solid background). According to Hypothesis II, F1 is involved in only
executing the premotor cortex instructed movements. LGM is based on the latter
hypothesis. The details of LGM are shown in Figure
6.4. Note that we introduced area F2 for complementing
the MNS structures. The visual input to area F2 originates from MIP (not shown)
and V6a
The alternative hypothesis is that learning to grasp circuit
is distributed over a large area in the premotor cortex including area F5, with
access to direct object affordance input from AIP; and area F1 is responsible
for the execution of the plan instructed by area F5 (see Figure 6.3). According to this hypothesis, the inability of
infants to perform adult-like grasps can be explained by the underdevelopment
of AIP for affordance extraction and the inability of infants to control their
limbs, or the computational complexity of AIP-F5 learning. The former
corresponds to the maturational-based theory of motor development; while the
latter corresponds to learning-based theories (see Chapter 5).
We will introduce Affordance-based Learning to Grasp Model
(LGM) based on Hypothesis II, which means that LGM will be the model of grasp
related visuomotor circuit of monkey premotor cortex. LGM, being a simulated
neural realization of Hypothesis II, will yield testable neurophysiological
predictions.
With the term learning to grasp we mean to learn how
to make motor plans in response to sensory stimuli such that the open loop
execution of a plan leads to a successful grasp. There is strong behavioral
evidence that early grasping is based on open-loop control and does not use
visual feedback (Clifton et al. 1993;
Clifton et al. 1994; von Hofsten and Ronnqvist 1988; Streri 1993). Further, adult practiced
movements move from a visual feedback control strategy to an open loop control
strategy evidenced by postural invariance studies in grasping movements.
Desmurget et al. (1998) studied a prehension task
requiring subjects to grasp a cylindrical object presented at different
locations with changing orientations. The effect of initial arm posture was
investigated. The results showed that individual subjects had stereotypical
grasping patterns resulting in fixed postures of the arm, which varied
systematically as a function of initial posture and object location and
orientation (Desmurget et al. 1998). Furthermore Grea et al. (2000) showed that the final posture
to be reached is planned in advance and used as a control variable by the
central nervous system. This was true even when the object jumped to a new
location during the transport phase of the reach. The new position of the
object could determine the final arm posture with the same precision as a
stationary target (Grea et al. 2000).
However, we do not claim that visual feedback is not used
for reaching and grasping. On the contrary, we suggest that when the task
demands cannot be satisfied with existing motor schemas, the visual feedback
control becomes necessary. In Chapter 7, though in a simplified setting, we
study the visual feedback control of grasping. Current chapter addresses the
issue of acquiring a grasp repertoire that can generate suitable grasping based
on the object affordances.
The link between open-loop grasp execution and the visual
feedback based grasp execution of Chapter 7 is established through AIP-F5
learning in LGM. As will be shown LGM learning yields units that show object
selective responses similar to F5 canonical neurons. The favored hypothesis of
Chapter 7 is that F5 canonical neurons, based on the object properties, gate F5
visual servo circuits. Thus, Chapter 7 without loss of generality, will
concentrate on a visual servo circuit specialized for precision grip learning
and execution, which is presumably selected by a population of F5 canonical
neurons.
We propose three computational layers relevant for grasping:
Hand Position, Virtual Finger, Wrist Rotation layers, as we functionally
justified in Chapter 5. The affordance of the target can vary from a single
variable indicating the existence of a graspable object to the full description
of an object in terms of its affordances, and is encoded in the Affordance
layer. The affordances are represented using the population-coding scheme and
encoded algorithmically (i.e. no visual processing for object recognition and
feature extraction is performed). We use Affordance layer or the input
terms interchangeably to conform the context
The parameters (hand position, virtual fingers and wrist
rotations) encoded in LGM layers are abundantly represented in the premotor and
motor cortices. Thus, it is not always possible to constrain the localization
of the layers with a high level of confidence. Nevertheless, we can minimize
the number of alternatives based on relevant literature as follows.
The wrist rotation parameters are represented in the
primary motor cortex (area F1) in terms of direction (i.e. independent of the
muscle groups activated) (Kakei et al. 1999). The ventral premotor cortex
(area F5) neurons are involved in extrinsic coding of hand direction (Kakei et al. 2001). Area F2 has control
over wrist movements and is organized similarly to area F1 in terms of
somatotopic organization (Fogassi et al. 1999); thus Wrist Rotation layer
can be associated with F2 as well as with F1. Premotor cortex (area F4) can
potentially encode hand location with respect to the object (Fogassi et al. 1992;
Fogassi et al. 1996),
as it is the target of ventral intraparietal area (VIP) (Geyer et al. 2000), which is involved in
egocentric target representation (Duhamel et al. 1997). The VIP-F4 circuit,
therefore, can play an important role in reach and grasp planning in monkeys (Rizzolatti et al. 1998). Thus, it is tempting to
associate the Hand Position layer with area F4 and/or area F5. Recalling the
object selective motor properties of F5 neurons (see Chapter 2), we can posit
F5 in performing the task of Virtual Finger layer by instructing which
configuration to use for a given object. In addition, Cisek and Kalaska (2002) presented evidence that the
primate premotor cortex can simultaneously represent discrete directional
signals related to multiple alternative reaching actions. In this study, a
monkey was asked to reach for possible targets, which were cued by a nonspatial
‘go’ stimulus. During the first period, while the monkey was waiting for the go
signal, two directional signals coexisted in the activity of neurons in dorsal
premotor cortex encoding the reach directions toward the two potential targets (Cisek and Kalaska 2002). When the ‘go’ signal was
given, the activity encoding the non-cued direction disappeared and the
remaining signal predicted the monkey's reach choice (Cisek and Kalaska 2002). In a similar study by Hoshi
and Tanji (2000), an additional cue was
introduced to instruct the monkey to use its left or right hand when pointing
one of the two targets. The results indicated that alternative motor plans were
represented in the dorsal premotor cortex before the action is uniquely
determined. Note that one of the main motivations behind the neural
architecture we developed in Chapter 4 was to be able to encode multiple action
plans.
In the light of the above review and in accordance with
Hypothesis II, we propose that area F5 works closely with areas F4 and F2 to
create a feasible grasp plan based on the object affordances relayed via AIP
(see Figure 6.4). The grasp plan is then, relayed to the primary
motor cortex (F1) and the spinal cord for execution. The tactile feedback of
the grasp is assimilated in the first somatosensory cortex (SI), which mediates
learning in parietal and premotor connections.
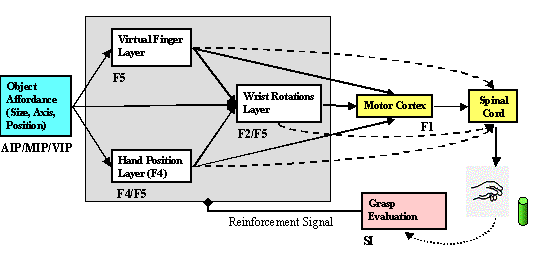
Figure 6.4 The
Learning to Grasp Model. F5 is implicated in all grasp related parameters.
Dashed connections indicate the direct corticospinal projections of premotor
areas. Area F5 works with area F2 and F4 to transform visual affordances
signalled by parietal areas into a grasp plan. The grasp plan is then, relayed
to primary motor cortex (F1) and spinal cord for execution. The tactile
feedback of the action is integrated in the first somatosensory cortex (SI),
which mediates the adaptation of the parietal-premotor and inter-premotor
connections
The involvement of area F5 in multiple grasp parameter
coding finds support by the finding that most F5 neurons were selective for
grasping movements, but there were also reach related as well as wrist rotation
selective neurons (Rizzolatti et al. 1988). The multiplicity of motor
representations (Wu et al. 2000;
Rizzolatti et al. 1988; Gentilucci et al. 1988) makes it impossible to rule
out other alternatives such as the view that F5 being exclusively involved in
mapping object affordances to finger configurations. This, in fact, points out
the importance of our modeling. With our model, we aim to motivate
neurophysiologists to challenge the model predictions with experiments, which
will help uncover the detailed functional roles of the premotor areas in grasp
related visuomotor transformations.
When a human infant or a monkey touches, many receptors in
the hand transmit many signals related to the contact such as the skin
indentation and slip (Rothwell 1994;
Johansson and Westling 1987b; Johansson and Westling 1987a; Salimi et al.
1999b).
The literature on the mechanisms of mechanoreceptors and the transmission of their
signals to the cortical areas is vast. Our intention is not to model these
mechanisms, but rather convince the reader (and ourselves) that a reinforcing
signal indicating a successful grasping is available to the learning-to-grasp
circuit located in the premotor cortex. Thus, we present here a brief but
relevant data from the literature on the role of the somatosensory cortex in
representing the sensations of the hand, including grasping. The interested
reader is referred to other literature (e.g. Akoev et al. 1988;
Willis and Coggeshall 1991) for detailed information on mechanoreceptors and
their functional organization.
The primary somatosensory cortex (SI) has somatic
representation of the fingers and shows differential activity during grasping
phases (Gardner et al. 1999; Ro
et al. 2000).
In one study, anterior SI was found to be dominated
almost exclusively by neurons with cutaneous receptive fields (88%) and
posterior SI neurons were found to receive tactile inputs (51%) and deep inputs
from muscle and joints (41%) (Debowy et al. 2001). Furthermore, Debowy et al. (2001) showed that during
prehension somatosensory cortex units signaled the formation of hand and
object as a functional unit in combination with other hand actions. Debowy
et al. (2001) classified SI neurons,
among others, as approach, contact, contact-grasp, grasp-lift, manipulation and
grasp inhibited neurons. In terms of tuning, they have found neurons with grasp
tuning, approach tuning, hold tuning and contact tuning.
Martin et al. (2000) examined the effects of
blocking neural activity in cat sensory motor cortex (muscimol infusion) during
early postnatal development on prehension. Grasping occurred on only 14.8% of
trials with the limb contralateral to the infusion. In addition, the grasping
was replaced by raking without distal movements. This data suggests that the
normal development of skilled motor behavior requires activity in the sensory
motor cortex during early postnatal life.
Salimi et al. (1999) examined the receptive field
properties of somatosensory cortex neurons in monkeys during a precision grip
task. The majority of the receptive fields found was cutaneous and covered less
than one digit. Two types of neurons were described: dynamic and static. The
dynamic neurons, showed a brief increase in activity beginning near grip onset,
which quickly reduced even the pressure to the receptive field continued (Salimi et al. 1999a). Some of the dynamic neurons
responded to both skin indentation and release (Salimi et al. 1999a). The static neurons had
higher activity during the stationary holding phase of the task (Salimi et al. 1999a).
Based on the brief review above, we postulate that grasp
success is signaled as a population activity in the somatosensory cortex (SI).
In order to simulate and test the proposed grasp-learning hypothesis we need to
emulate the grasp success signal to drive the learning in LGM. We base the
emulation on the physical definition of grasp stability as we did in Chapter 5.
Of course, we do not claim that such computation is performed in the primate
brain. We are using the tenets of schema methodology (Arbib et al. 1998) to substitute a biological reinforcement
or success schema (‘joy of grasping’ of Chapter 5) with the engineered
version for the sake of analysis.
We have already introduced LGM layers in Chapter 5 as
schemas. Now, we summarize the functional description of these layers to
prepare the reader for neural level analyses.
Hand Position layer determines where the hand will be
with respect to the object during grasping. Intuitively, this represents the
side from which the hand will grasp the object. The frame of reference for this
parameter is allocentric. The coordinate system we choose for this parameter is
spherical. The encoding used is as described in Chapter 4. Thus the neural
layer represents a probability distribution of hand position values and the
specific output is generated by a local population vector computation.
Virtual Fingers layer specifies which fingers will be
activated, and with what strength. We use three virtual fingers: thumb, index
finger, and the remaining three fingers acting together. The processing is
parallel to the Hand Position Layer’s flow. However, this does not mean that
this layer has the right to decide the virtual fingers on its own. The virtual
fingers that cannot yield grasping are negatively reinforced; and hence do not
appear in learned LGM.
Wrist Rotation layer combines all the information
about the object (affordance) and the Hand Position and Virtual Finger layers’
output. Thus, the output of this layer represents the possible wrist
orientations given the (1) object related input (affordances), (2) output of
Hand Position layer (3) output of Virtual Fingers layer in terms of a
conditional probability distribution. The parameters generated in this layer
determine the movements of wrist extension-flexion (tilt), wrist
supination-pronation (bank); and ulnar and radial deviation (heading).
A typical scenario for grasp execution would be as the
following:
·
A small box is presented in the workspace of the arm to
the left of midline of the body.
·
Hand Position layer computes the distribution of
feasible approach directions and a selection is made according to the
distribution, for example as ‘from top’
·
The Virtual Finger layer works similarly. Let us assume
that a selection is made such that index and thumb fingers are activated such
that their trajectories coincide (a precision pinch).
·
Then, the Wrist Rotation layer combines the Virtual
Finger and Hand Position layers’ parameters with the Affordance layer to
compute a probability distribution for the applicable (e.g. to the precision pinch approaching to the object from top)
wrist orientations. The grasp plan is complete when the final selection is made
from the Wrist Rotation layer.
Microstimulation studies ensure that there are neurons in
the primary motor cortex and premotor cortex that control finger digits, wrist
movements, and reaching. This chapter provides a learning mechanism to adapt
the connectivity of premotor regions so that they act cooperatively to yield
feasible grasp plans. One of the main motivations of using a minimal set of
grasp parameters was to test whether learning by interacting with the
environment, can shape neurons to have properties that we did not manually
encode. The emergent neuron properties such as the object selectivity are very
important because they justify that the structure and the learning proposed are
adequate to capture the learning for the grasp related visuomotor
transformation in the primate since we bootstrapped the grasp learning from a
minimal set of elemental/postnatal abilities and behaviors summarized as the
following.
1.
Motor abilities: infants are able to move their wrist, and
fingers
2.
Visuomotor abilities: infants reach for visual targets
3.
Behaviors: infants explore the space with their hands through
variable movements
4.
Reflexes: infants are born with reflex behaviors helping them
to shape their (visuo)motor abilities (e.g. enclosure reflex)
This is why we did not assume, for example, a layer of grasping
neurons specialized for different hand apertures. If we did so, those
neurons and the objects with compatible sizes would be trivially associated via
trial and error learning, which would not add anything to our knowledge.
However, with our approach we show that starting from a basic set of
abilities/behaviors in accordance to infant development, complex neural
properties emerge which yield predictions that can be experimentally tested. If
the model is validated by comparing experimental findings to predictions of the
model, we can suggest that primate brain follows a similar strategy to develop
visuomotor abilities by interacting with the environment. Thus, a validated
model will (1) bring new insights to primate visuomotor transformation and (2)
enable us to make sound predictions with new simulation experiments. As will be
presented in later sections, LGM leads to the emergence of neurons with
nontrivial properties such as object preference, similar to those of F5
canonical neurons in monkey premotor cortex.
Chapter 5 showed that Infant Learning to Grasp Model (ILGM)
was able to learn how to adjust wrist orientation and approach-direction via
explorative learning. The model learned to generate parameters to perform power
and precision grasps, and many variations of the two. The most abundant grip
generated was the power grip and its variations. Precision type grasps were
less frequently generated. In terms of learning task, the ILGM had to discover
the distribution of wrist movements (supination-pronation, extension-flexion,
ulnar/radial deviation) for multiple approach directions. Presented with an
object, Hand Position layer produced the distribution of possible approach
directions. The selection was relayed to Wrist Rotation layer. Then, Wrist
Rotation layer computed the distribution of feasible wrist orientations
conditioned on the generated hand position. Virtual Fingers layer dictated the
enclosure speed of the hand.
Noting that ILGM being functionally equivalent to
LGM, analyzed the behavioral aspects of grasp learning, we now present a neural
level analysis of the wrist-orientation learning of Chapter 5.
In this section, we analyze the activities of LGM layers. We
will use two graphical representations: one for the probability distribution
represented by LGM units and one for the memory (or eligibility) traces. The
former map represents how likely the units would fire given the input and the
context while the latter represents the ‘generated parameter’ at the particular
instant. A neurophysiological analogy for the probability distribution would be
a multi-electrode recording experiment. The normalized firing histogram of the
neuron population over many trials (with fixed input and the same experimental
conditions) would be very much comparable to what we call the probability
distribution. Thus the probability distribution graphs represent (1) population
level activity of the neurons in a layer and (2) individual neurons’ preferred
stimulus. The eligibility trace maps show the activity of neurons that are
involved in parameter generation in a single trial. Figure 6.5 shows the activity of the Hand Position and Wrist
Rotation layers (left two panels) as well as the memory traces (center two
panels) for the grasp shown in the right panel. Note that the preferred values
of Hand Position and Wrist Rotation layers are three-dimensional parameters. To
visualize them we average the activities over an axis. For Hand Position we
average over radius axis. For Wrist Rotation layer we average over the ulnar/radial
deviation axis. These two axes are chosen because they are found to be
least influential in generating successful grasps.
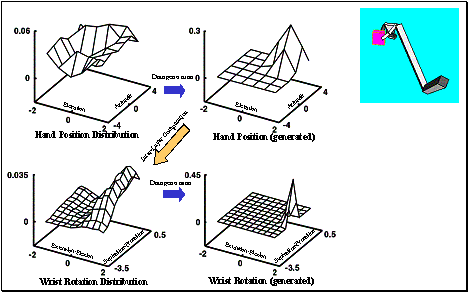
Figure 6.5 The
top-left shows the Hand Position layer output summed over the radius (approach
direction is encoded in spherical coordinates) as a 3D plot. The top-centre panel shows the sample
generated from the Hand Position distribution. Bottom-left shows the Wrist
Rotation layer output summed over the heading axis as a 3D plot. The bottom-centre
panel shows the parameters picked from the Wrist Rotation layer distribution.
Note that Wrist Rotation layer distribution depends on (i.e. represents a
conditional distribution) the sample picked from the Hand Position layer. The
rightmost panel shows the executed grasp
The allocentric position encoded by top-center map roughly
corresponds to a location higher than the object, which is also at the behind
and right side of the object. This location determines the approach direction
of the hand (hand reaches for the object from that location). Given this
approach the possible wrist rotations that yield stable grasps are computed by
Wrist Rotation layer. The bottom-left panel shows the firing potential of units
in this layer. Having a population level activity, we can interpret the
strategy ILGM learned. The Wrist Rotation probability distribution roughly
says, as long as the wrist is flexed between 30 to 80 degrees (tilted downward)
then the grasp will succeed with the hand approach direction encoded in the
memory trace. Of course, there is no way to tell in advance, which of the
combinations will yield a precision pinch. Our general notion is among the
performed grasps, there will be precision grips, which will be picked and tuned
by the premotor cortex.
Now we look at another grasp plan made by the same LGM (no
new training). As we mentioned earlier Wrist Rotation layer learns feasible
wrist rotations based on the eligibility trace of the Hand Position layer. This
time Hand Position layer generated a different approach (Figure 6.6, top-center panel), which changed the landscape of
the Wrist Rotation layer’s distribution (bottom-left graph). The wrist rotation
parameter generation is shown in the bottom-center panel and the resulting
grasp is shown in the right panel.
One of the important observation is that LGM was able learn
a ‘menu’ of grasps which is open to biasing. The bottom-left panel of Figure 6.6 explicitly shows that two distinct sets of wrist
rotations could be generated given the approach direction encoded in the memory
trace of the hand position parameter (Figure
6.6, top-center graph).
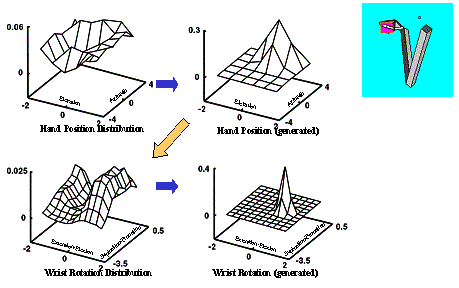
Figure 6.6 Using
the same LGM used for Figure
6.5, another grasp plan is generated (left four panels).
The resulting grasp is shown on the right. By comparing the grasp plan shown on
the left four panels with of Figure
6.5’s grasp plan we see how the selection of a different
approach direction (see the centre-top panels of both figures) changed the
Wrist Orientation distribution
Now we demonstrate the two options from the ‘grasp menu’. Figure 6.7, shows clearly the variability of the grasp that can
be encoded in LGM. In the upper panels the Wrist Rotation layer specified
maximum wrist extension with pronation (top-center panel). The resulting grasp
is shown on the upper-right panel. In the second trial, the generated wrist
rotation instructed maximum supination and small extension. The resulting grasp
was very different although the hand approached the object from the same location
(the Hand Position layer is not shown).
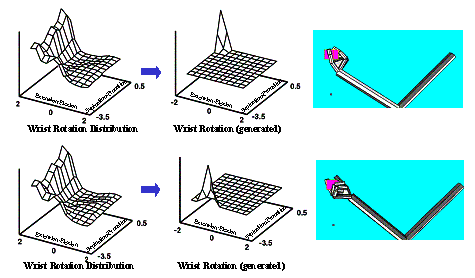
Figure 6.7:Two very
different grasp generation from the same LGM. Upper panel: Grasping with
maximum wrist extension with some pronation. Lower panel: Grasping with maximum
wrist supination and small wrist extension. Note that the Wrist Layer
probability map is the same since the approach direction was chosen the same
(the small dots in the right most panels).
LGM simulation in particular predicts that the premotor
cortex neurons that are involved in encoding limb movement parameters (e.g.
wrist rotations) must be modulated by other movement parameters (e.g. the
direction of approach).
By generalizing grasp actions to general movements, we
predict that complex movements cannot be determined by a single layer. For
example, the neurons controlling digits and wrist rotations are segregated in
area F2 (Fogassi et al. 1999); area F4 controls proximal
while area F5 distal movements (Rizzolatti et al. 1988;
Gentilucci et al. 1988).
Somatotopic organization of multiple motor areas is an evident strategy of the
primate motor cortex (see reviews: Wu et al.
2000; Geyer et al. 2000).
Thus, the general prediction we make is that the activities in these segregated
regions must be modulated by their related peers (e.g. hand-arm). For an
experimenter the neurons of this nature would appear to have ‘gain fields’ or
appear to be modulated by other behavioral contexts.
Let us take a hypothetical but common experimental
set up (for example see
Georgopoulos et al. 1982).
The experimenter wants to find the neurons encoding wrist extension-flexion in
monkey. The monkey places its arm on a table and performs wrist extension and
flexion movements. The experimenter locates the motor region that (e.g. F2)
correlates very well with the monkey’s movement. Can he say that this region
encodes wrist extension/flexion? With the insights gained from our simulation
studies, we answer ‘no’. The activity could be well depend on other motor
parameters, such as posture. Indeed, this kind of modulation has been shown to
exist in the primary motor cortex (Sergio and Kalaska
1997)
with reaching movements using different arm postures. The finding has also been
extended to the dorsal premotor cortex confirming that similar modulation
exists in higher motor areas (Scott et al. 1997).
In fact, Arbib and Hoff (1994) noted the important
distinction between neural activity that controls movement versus neural
activity that correlates with movement. We propose that to discover
motor circuits in the cortex the correlation studies (correlating behavior to neural
firing) is not enough. Simultaneous recording from anatomically connected
regions must be required to understand the computational elements underlying
the modulation or gain field phenomenon
Lockman et al. (1984) used subjects of 5- and
9-months of age and compared their performance in orientating their hands to a
dowel presented in horizontal and vertical position (see Figure
6.8).
To replicate their experimental condition, we let the affordance of the object
(orientation) to be relayed to Learning to Grasp Model in accordance with our
Hypothesis II (see Figure 6.3). We presented a cylinder to LGM analogous to a
dowel. To implement the premises of our hypothesis that the information about
the axis orientation (i.e. affordance of the object) is not available to the
grasp planning circuit during early infancy (5 months of age) and that it
becomes available later in development (9 months of age), we effectively
disabled the orientation encoding for the simulation of younger infants’ grasp
learning whereas to simulate older infants’ case we enabled the orientation
coding in their affordance input (see Chapter 5). The former case was referred
as the poor-vision and the latter as the full-vision condition.
With this simulations we had two goals: (1) to compare the
simulation results with of Lockman et al.’s (1984) and (2) to analyze the
internal representation of the learned model. Chapter 5 compared the model
results with experimental data fulfilling (1). Now we present the internal
representation analysis of LGM for both affordance and no affordance case
representing 9 months and 5 months of age infants respectively.

Figure 6.8 The
grasps performed after LGM learned the association of hand rotations with the
object orientation input (full vision condition). Note that the left panel
shows a bottom side grasp. All of the shown grasp configurations satisfied
grasp stability criterion
By analyzing the kinematics error curves of the orienting
behavior, we concluded that there were two modes of grasp planning (Chapter 5);
but how correct is this? We answer this question by examining the population
level representations emerged by LGM learning.
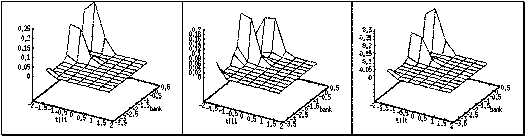
Figure 6.9 In the poor-vision case,
the hand rotation neurons in LGM show the same response for horizontal (left
panel), diagonal (centre panel) and vertical (right panel) object presentations
because of the lack of axis orientation input
We are interested in deciphering the grasp planning strategy
learned by LGM in poor-vision condition. Figure
6.9 shows the Hand Rotation neurons of LGM: (from left to
right) for horizontal, diagonal and vertical cylinder presentation. The Hand
Rotation distribution is almost identical since the axis orientation
information is not accessible to LGM in the poor-vision case. The Hand Rotation
Layer distribution confirms our inference from the kinematics: there are two
peaks of neuron activity, which corresponds to the vertical and horizontal
orientations of the hand during grasping.
However, note that the two possibilities could not be specialized
for the different orientations and hence represented in all three cases. The
peak at (-p/2, 0) indicates a wrist rotation that makes the angle between the backside
of the hand and the arm 90 degrees (full wrist extension), which is required
for grasping a centered horizontal object from the front side. On the other
hand, the other peak at (-p/2,p/2) indicates an additional supination of the hand, which is required for
grasping vertical cylinder from the front side. These values corresponds to the
grasp configurations we kinematically observed in Chapter 5.
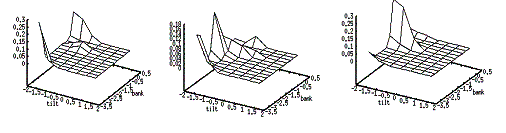
Figure 6.10 When
LGM has access to axis orientation information the Hand Rotation neurons
represent different plans in response to horizontal (left panel), diagonal
(centre panel) and vertical (right panel) object presentations
In the full-vision
case, we supply the orientation of the cylinder as an input to the LGM. Figure 6.10 shows the Hand Rotation neuron’s distribution for
different orientations, for a direct comparison with poor-vision case Figure 6.9. Now the neurons show preferential activity
for different cylinder orientations. Also we can see that in the horizontal
orientation case the LGM have two alternative plans as evidenced by the two
peaks at the edges of the Hand Rotation neuron distribution shown in Figure
6.10, left panel. Note that these two alternatives
correspond to grasping the horizontal cylinder from top and bottom (left panel,
Figure 6.10).
Similar multiple plan
representation is also observed in the diagonal case (Figure 6.10, center panel). The vertical cylinder case has a
single grasp plan representation (Figure 6.10, right panel). We can relate the Hand Rotation neuron
activities of poor-vision and full-vision cases. We can see that the
poor-vision distributions are superimpositions of the three maps shown in Figure 6.10 with varying degrees of inhibition on areas where a
common activity was not observed. For example there is activity around (-p/2,-p/2) in all three neuron responses (Figure 6.10), and hence the peak appeared in the responses shown
in Figure 6.9. However, the peak of activity (-p/2, -p), which is shared by horizontal and diagonal
orientations is not pronounced in Figure 6.9, because these neurons are not active when the object
is vertically oriented (Figure 6.10, right panel)
Based on the wrist
rotation and hand position maps formed via learning we can make predictions
concerning the neural response properties of the premotor cortex. Up to now, we
have used the term Affordance or Input to LGM without specifying
from where this information can be relayed to premotor regions. Now we relate
the emergent properties of the simulated neurons to affordances relayed.
The anterior part of the lateral bank of the intraparietal
sulcus (area AIP) is implicated in extracting visual properties of objects
relevant for grasping (Sakata et al. 1997a;
Sakata et al. 1998; Sakata et al. 1995; Murata et al. 1996). There is strong anatomical
reciprocal connection between area F5 and area AIP (Matelli 1994, Sakata,
1997). Furthermore some AIP visually activated neurons, show tuning according
to the orientation of the longitudinal axis or the plane (surface) of flat
objects (Sakata et al. 1999). Therefore, the axis
information can be extracted by area AIP and channeled to area F5. The lateral
bank of the intraparietal sulcus (c-IPS area) is involved in three-dimensional
analysis of objects (Sakata et al. 1997a;
Sakata et al. 1999).
Some of these binocular visual neurons are selective for the orientation of the
axis of the objects (AOS neurons). The c-IPS neurons may be the basis of AIP
neuron properties (Sakata et al. 1997a;
Sakata et al. 1999).
Analogous to the AIP-like object representation found in
area F5 (Murata et al. 1997a), our model predicts that a
premotor area that is connected with AIP or area F5 must have visually
triggered neurons that are selective for object orientations.
Furthermore, we suggest that the area must have control over wrist rotations.
Area F5 and F2 of the dorsal premotor cortex, satisfies the conditions we have
listed. Fogassi et al. (1999) found that F2 neurons
responded to visual stimuli and showed by microstimulation that the wrist
joints were controlled within area F2. To our knowledge there is no study
probing the object orientation selectivity of neurons in area F2 and F5. We
suggest that the canonical neurons may have tuning fields according to the
object orientation similar to the object type neurons with orientation tuning
in area AIP (Sakata et al. 1999).
To summarize, we predict that a sub-population of F5
canonical neurons or F2 neurons that are involved in wrist control must have
selectivity or tuning according to objects’ grasp relevant properties. More
specifically, we claim that a closer examination of F5 canonical neurons and/or
area F2 neurons would reveal orientation selectivity either in isolation
or as a tuning mechanism over object selectivity.
The goal of this simulation is to investigate the emergent
neural properties when Learning to Grasp Model is presented with objects of
different sizes. The object size is accessible to LGM via the Affordance layer
and algorithmically coded (no object recognition and feature extraction is
performed).
We used cubes of different sizes as objects. After the model
interacted with the object, we identified the grasps learned. For large object
case, the model often generated power grasps, and some variations. For small
objects model generated precision grasps. The number of different precision
grasp types was less compared to the number of different type of power grasping
observed for big objects. In other words, LGM formed a large ‘menu’ of power
grasps but gained a small ‘menu’ of precision grasps.
After the behavioral observation, we analyzed the unit level activity of Hand
Position layer. Wrist Rotation layer’s output was modulated by Hand Position
layer’s activity (see the analyses in section 6.6).
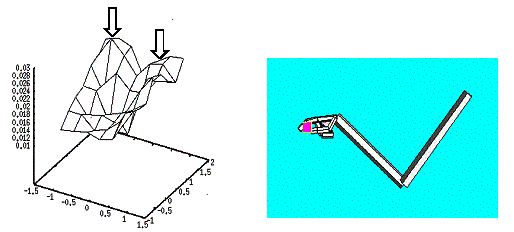
Figure 6.11 The
small object presentation produced two peaks of activity in the Hand Position
layer corresponding to the probability distribution of approach directions. The
right panel shows the executed grasp when the data generation was localized in
the area pointed by the leftmost arrow.
Figure 6.11 shows a learned precision pinch and the activity of
Hand Position layer. The small object activated two peaks as indicated by the
arrows in the figure. If a neurophysiologist recorded the activity of the units
in Figure 6.11 (each point on the 3D mesh represents a neuron with
its average activity as the z-axis), he would notice over many trials that
those units are the most active ones. To claim the specificity he still would
need to compare the very same neuron with different object sizes. We do the
same and show that the activity is object-size specific (compare with Figure 6.12 and Figure
6.13).
Figure 6.12 shows a power-like (radial palm grasp see Figure 5.1) grasp with the corresponding Hand Position layer
activity. This time the hypothetical experimenter would notice the unit
indicated with the arrow fired maximally for the presentation of this object.
Note that the activity locus is different from the small object presentation
(see Figure 6.11).
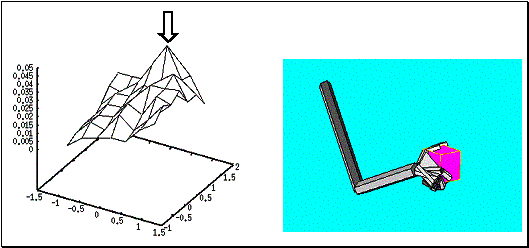
Figure 6.12: A
large cube was grasped by securing the object between the thumb and the other
fingers (right panel). The Hand Position layer activity is shown on the left
panel. The neuron with largest activity is marked with an arrow
As the last simulation we present the unit level activity
when the model presented with the largest object. The Hand Position layer
showed a clear peak (Figure 6.13). In order to emphasize that these simulated neurons
do not show unspecific activity, but rather are selective for object sizes, we
compare the locus of the neurons that the arrows point to in each figure (Figure 6.13;Figure
6.12; and Figure
6.11)
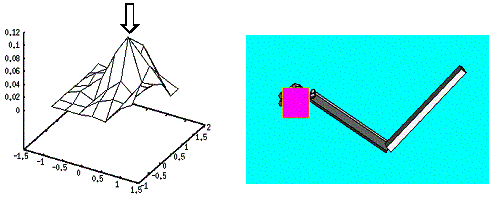
Figure 6.13 The largest
object presentation and grasping. The Hand Position reflects a single reach
direction as indicated with an arrow
Figure 6.14 shows the three activities superimposed after the
axis are aligned. The maximum activity regions are also linked to the
corresponding object presentation. Notice that the peak activity loci are not
the same indicating that the neurons gained selectivity to object size.
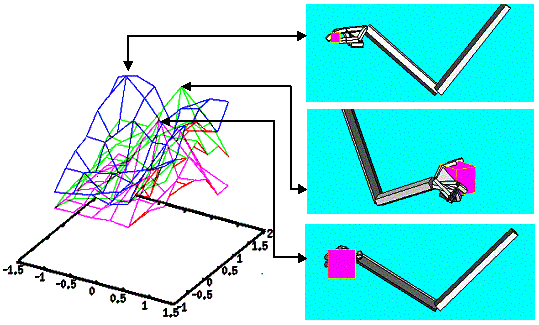
Figure 6.14:The
Hand Position layer activity is superimposed to demonstrate that the maximum
activity loci are separated for each object indicating selectivity for
object size.
Even though we did not engage Virtual Finger layer in its
full capacity (it controlled the fingers synergistically) the Hand Position
layer activity of LGM showed object specificity. Therefore, we suggest new
experiments to test whether F5 canonical object specificity is due to some
other motor parameter encoding. Although microstimulation of F5 neurons
produces complex movements they do not produce a grasping behavior, so the
activity during grasping do not necessarily specify complete grasp plan but
rather a subset of motor parameters required for grasping. This is analogous to
the LGM layers differential contribution to the grasp plan. Therefore, the
challenge to the experimentalists is to find out whether F5 canonical activity
alone specifies a grasp plan or not. Our prediction is negative because, for
example, in LGM simulations, the wrist rotations generated did not affect the
activity of Hand Position neurons (analogous to F5 canonical neurons’ object
related activity) but the grasp plan was only complete with the Wrist Rotation
layer’s contribution. Thus, F5 canonical neurons can be just a part of the
grasp planning circuit.
To decipher the functional structure of the premotor
grasping circuit, this hypothesis must be either ruled out or validated. If it
turns out that F5 neurons are specifying only a subset of grasp parameters, new
experiments must be designed to uncover the neuron properties of the areas
connected area F5. Since our model predicts that if some neurons specify a
grasp plan partially (by being responsible for one parameter of the plan), the
learning must shape the connections between the connected regions to enable the
cooperative computation that can yield compatible grasp parameters. This is a
nontrivial observation. if a hypothetical experimenter, investigating the encoding
of grasp parameters, finds out that
1.
Area A is responsible for wrist orientation
2.
Area B is responsible for controlling the reach direction
3.
If the activities of A and B are true control variables of
wrist rotation and reach direction
Then, we claim that
1.
Regions A and B must be anatomically connected (the connection
may be a multi staged one, such as A connected to C, C connected to B)
2.
The synaptic strength of the connections between A and B
encode the grasp selection strategy of the grasping circuit
3.
If the parameters are, in the physical sense, coupled then the
dependency of parameters must be captured in the connection between A and B
The claim (3) is based on the observation that the only way
to produce coherent grasp plans is to capture the physical coupling inherent in
the movement. For example given a sphere, there are infinitely many approach
directions, which can be used for grasping (assume A encodes approach
direction). Similarly, there are infinitely many wrist orientations (assume B
encodes wrist orientation). The crucial observation is that, not all pairs of
approach direction and wrist orientation are compatible. For grasping, given A,
B has to be determined or given B, A has to be determined because the
parameters are coupled. With this setting, we further claim that:
(1) If it is found that the connection between A and B is
unidirectional (say from A to B) then (from the view point of the experimenter)
the activity of B neurons must be tuned by the population activity A
(2) Recording simultaneously from A and B can reveal the
organization of visuomotor grasping circuit as the following. If activity of A
predicts the activity of B, better than the other way round (i.e. activity of B
predicts the activity of A) the underlying principle is that area A selects
some of the grasp parameters and then B, based on the selection of A, generates
the remaining grasp parameters
In terms specific predictions, LGM predicts that F5
canonical neurons, to specify a grasp plan, must have ‘gain fields’ based on
other brain areas (which, behaviourally would appear to be based on other
movement parameters). A good example for such systematic relation
(unfortunately, to our knowledge only behaviourally) is the effect of arm
posture on reach-related activity of motor cortex neurons (Caminiti et al. 1990;
Caminiti et al. 1991; Sergio and Kalaska 1997). In these studies, it has
been found that the reach encoding neurons have gain fields based on arm
posture or position of the arm in the workspace. These findings, combined with
multiple action representations in the motor areas (Cisek and Kalaska 2002) strongly indicate that the
underlying circuit for reach generation have similar properties as LGM.
The structure (the dependence relations of layers) we
offered in LGM may not be the only possibility. To uniquely determine the
structure, neurophysiological studies must go beyond correlation studies. LGM
postulates the dependency of wrist rotations on the virtual fingers and
approach direction. Therefore, according to LGM, virtual fingers and approach
direction predicts the wrist orientation. Then LGM predicts that the wrist
orientation coding neurons will have ‘gain fields’ based on the virtual fingers
selected and the approach direction determined. Whether LGM structure is right
can be tested by experiments similar to reach and posture coupling experiments (Caminiti et al. 1990;
Caminiti et al. 1991; Sergio and Kalaska 1997; Scott et al. 1997).
An alternative structure for LGM would be to posit that the
wrist rotations with virtual fingers are determined first then based on those
the approach direction is determined. This can be a feasible alternative
because the wrist orientations are important in determining the manipulative
freedom of the hand once the object is secured. For example when grasping the
knob of a door with the intention of opening, we intentionally choose a ‘hard’
wrist orientation to have a large manipulative freedom (i.e. turning the knob
to open the door). However, if we assume that the intended future action plans are
also relayed to wrist rotation layer (together with affordance of the object),
LGM structure can account for such anticipatory grasp planning as well.
LGM presents a rich set of hypotheses that can be verified
or falsified with clean-cut experiments. We now, suggest that simultaneous
recording experiments for premotor regions of F2, F5 bank (canonical neurons),
F4 and wrist rotation encoding neurons of motor cortex (F1) must be performed
to understand the underlying principles of grasp related visuomotor
computation. A feasible experimental setup would pick two pairs from (F2, F4,
F5, F1) at a time and record from the two sites while the monkey is performing
grasp actions directed to objects located at different positions with changing
orientations. The simultaneous pair-wise recording of areas F2, F4, F5 and F1
would require 6 sessions (F2- F4, F2-F5, F2 F1, F4-F5, F4-F1, F5-F1). We claim
that, with rigorous analyses of these recordings with reference to hand
kinematics and object affordances (i.e. the location and orientation of the
object, and the monkey’s wrist rotations and approach direction) the structure
of the cooperative computation underlying grasping can be revealed.
Up to now, we presented
simulations where the objects were located at a fixed position in the space. In
general, wrist rotations and approach directions depend on the position of the
object as well as intrinsic object properties. Here we concentrate on the
object location, but the analysis is valid for object properties (as long as
they are represented as population coded activities and relayed to LGM) such as
the orientation of the object. In fact we had already used multiple orientation
learning when simulating Lockman et al.’s (1984) experiments (sections 5.9 and 6.7). However, there, we did not perform extensive
analysis to show the generalization of the learning to novel orientations.
A given wrist rotation
and approach direction that yield a stable grasp, in general, does not
necessarily yield a stable grasp when the object is moved to an other position
as approach direction and wrist orientation parameters are coupled for a
successful grasp. For example, we don’t grasp objects that are located on the
left of midline as the same way as we do when they are on the right side. In
addition, reach component of different grasp actions yield different absolute
wrist orientations because the absolute wrist orientations depend on the
configuration of the arm. Thus, the grasp plan has to take into account the
location of the object. In this section we presents results showing that LGM
can learn to generate stable grasp plans for different locations and generalize
well for the locations that has not been experienced before. The generalization
property is important from a neural network perspective. A generalizing network
does not need to memorize all possible grasp plans for each location and
hence does not fail for novel situations.
We trained LGM model by
randomly placing a sphere in the workspace and letting
the model interact with the object. The egocentric location of object is
encoded algorithmically in the Affordance layer using population coding. Note
that there is evidence that objects are encoded in egocentric reference frames
in the parietal cortex (Siegel 1998; Colby and Goldberg 1999) which projects to premotor cortex (Geyer et al. 2000).
After 10000 grasp
attempts, the model acquired the ability to make grasp plans for objects
located in the workspace. Figure 6.15 illustrates the learning achieved as superimposed
images of completed grasps. Note that the object locations were not used while
the model was learning to grasp. The lower three grasp actions are anatomically
hard to achieve but they were included for demonstrating the range of actions
that LGM learned to grasp at. As in earlier simulations, the illustration in Figure 6.15 only depicts a possible grasp for each location out
of many alternatives. We reran the trained model on exactly the same object
locations as in Figure 6.15. A set of different grasping configurations is
selected to show that LGM could both generalize and represent multiple grasp
plans for each target location (see Figure 6.16).
Figure 6.15 The
trained Learning to Grasp Model executed grasps to objects located at nine
different locations in the workspace. The grasp locations were not used in the
training. All of the grasps shown were
stable
Figure 6.16 The same model used in generating Figure 6.15 was used to generate a different set of grasps. Again
all the grasps were stable.
The crucial feature of
LGM we implemented is that it can produce a variety of grasp plans based on
input. Furthermore, the layer structure is well suited for further refining or
biasing (e.g. contextual or motivational biases). For example, one can use
different biasing when grasping an apple for eating or for placing it in a
shopping basket. The reinforcement framework also gives the flexibility to
include soft constraints in grasp evaluation such that the grasps that are not
favorable (due to discomfort, excess energy consumption, etc.) are not
represented or represented with low probability. Thus the reinforcement signal
(‘joy of grasping’ of Chapter 5 and ‘neural grasp stability’ representation of
this chapter) can incorporate the anatomical and environmental constraints
which are important in shaping grasp development (Newell et al. 1989) or the adaptive value of Sporns et al.(1998)
We summarize the computational ingredients of grasp learning
we proposed with LGM by tracing the neural level computations through an
example.
We will use Figure 6.17 to illustrate how the same input condition can give
rise to different grasp plans. First, the object is presented (bottom center).
The object location is encoded in an egocentric reference frame using
population coding (we use a spherical coordinate system) (the center plot). The
object location is transformed into approach direction distribution (the
top-center) by Hand Position layer. After approach direction generation, the
center stream branches into two. On the left, the approach direction is
generated as (approximately) from bottom, indicated by the top-left plot. On
the right, the approach direction is even lower but it is from the backside of
the object as can be read-off from the top-right plot.
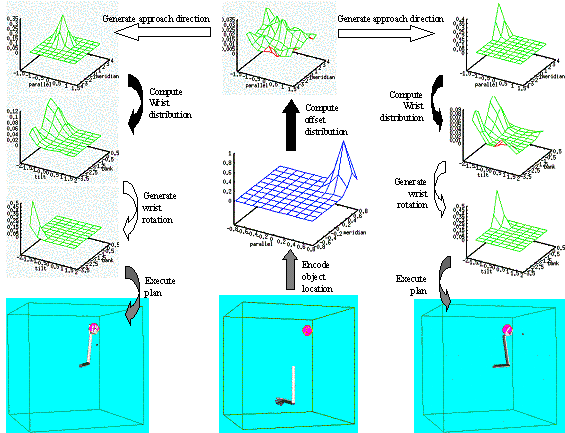
Figure 6.17 The
internal mechanisms of representing and generating multiple grasp plans are
shown. Solid arrows (except object encoding) denote learned connections while
empty arrows indicate data generation. The flow of operation starts with the
presentation of the object (the bottom centre) and follows the arrows. At the
top-centre, the data generation can yield multiple approach directions. The two
possible approach directions are shown creating two streams (left column and
right column), each of which yields different grasp execution (bottom pictures
of left and right column).
Based on this two
approach directions two different wrist rotation distributions are computed by
the Wrist Rotation layer as indicated by the downward solid arrows on each
side. This is followed by wrist rotation generation, which results in different
wrist joint rotations in the left and right streams as indicated with the
downward empty arrows. The grasp actions that are instructed by the left and
right grasp plans would yield the completed grasps shown on the bottom-left and
bottom-right columns respectively.
It has been argued that
mirror neurons forms the basis of understanding other’s actions (Gallese et al. 1996; Rizzolatti et al. 1996a;
Rizzolatti et al. 2001a; Umilta et al. 2001). The goal of this chapter is to bring an
alternative view that this cognitive function may be secondary to a role for
the mirror neurons in providing visual control signals for manipulation. Figure 7.1 shows the focus of this chapter. MNS model is redrawn
and the regions of interest are marked with the gray background rectangle. In
Chapter 3, we introduced the idea that the mirror neurons might be involved in
visual feedback for manipulation, but in implementation, we emphasized how the
self-action observations yield mirror neurons. In Chapter 6, we have
studied how grasp alternatives can be formed and selected based on the object
affordance and showed the emergent object feature selective properties of the
simulated premotor neurons. In this chapter, for the sake of tractability, we use planar arm/hand model and focus on
precision grasps. However, it should
be emphasized that the simplification we make does not reduce the value of the
message we wish to communicate to experimentalists. The point of this
chapter is not to show the object selective properties of the F5 neurons, but
to study the temporal aspects of grasping. Specifically, we show that units
that encode visually defined grasp errors can yield activities similar to
mirror neurons and suggest experiments to validate and challenge the visual
feedback control hypothesis of mirror neurons.
First, we present a
biologically realistic feedback circuit composed of leaky integrators
that can visually servo the hand to achieve grasping. Then we augment the
circuit with a feed-forward controller that is composed of pattern
matching neural units and present two alternative hypotheses that associate the
visual control signals with mirror neurons. We demonstrate that the visual
feedback and feed-forward grasping system can work with lower level motor
control circuits by implementing a position and velocity (PD) controller which
receives its desired trajectory from the visual grasp control model. Finally,
we introduce a method to compare the controller unit activities with real
mirror neuron recording data and suggest explicit experiments to validate or
invalidate our proposal that mirror neurons are involved in visual control of
grasping movements.
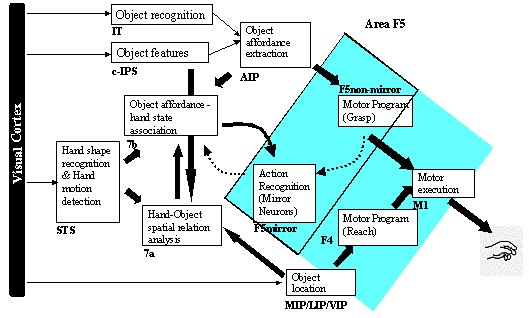
Figure 7.1 The MNS
model repartitioned to show the focus of this chapter. The grey background
marks area of interest
To our knowledge, up to now, all research on the mirror
system has focused on the operation of a mirror system with the implicit
assumption that it is developed for higher-level tasks (such as understanding
and imitation), without exploring the possible biological precursors. MNS
(Chapter 3) showed how observation of self-action may serve as the learning
stimulus for shaping the mirror neuron system but did not address the issue of
why the brain might contain such learning hardware. The standard answer is that
it is there to help the animal recognize the actions of others by means of some
similarity to its own actions. However, we wish to explore the hypothesis that
the mirror system can best be understood through exaptation of a system for
visual feedback control for manual actions.
For a reaching task, the simplest visual feedback is some
form of signal of the distance between object and hand. This may suffice for
grabbing bananas, but for peeling a banana, feedback on the shape of the hand
relative to the banana, as well as force feedback become crucial. The
parameters that are needed for such visual feedback have the ingredients of the
hand state we used in MNS. We do not claim that the hand state we defined
exists in the brain but we do claim that such hand-object relations must be
represented in the primate brain. There are studies where neurons sensitive for
observation of hands approaching to the points of attention or fixation have
been found (Siegel and Read 1997). Also there are studies
suggesting that the monkey parietal area have an allocentric representation of
object locations, which simply indicates that the distance between objects can
be encoded in parietal cortex (Murata 2000).
With Learning to Grasp Model (LGM) (Chapter 5), we showed
that it is possible to learn to generate grasping movements based on
available affordances. LGM is capable of generating precision grips, but it
does not have the machinery to visually servo the fingers to their targets on
the object. In other words LGM learns to generate grasp plans for open-loop
control. We suggest that a visual feedback system develops in area F5
augmenting the LGM grasp machinery. The fundamental idea is that LGM bootstraps
F5 visual servo system by presenting examples of suitable grasps that trains
the feedback system. In this chapter, we present two possible organization of
the manual visual servo circuit and perform simulations using one.
Here we do not use the control term strictly. It should be
understood that the command sent from area F5 are higher level than the control
command sent to actuators of a robot. In fact, in robotics the proposed area F5
output would be considered as a trajectory plan for grasping which
specifies the kinematics aspects of the grasp but leaves the dynamics to the
lower levels. In the next sections, we address the dynamics of the reach and
grasp by giving a 2D model of grasping. It is known that F5 projects directly
via the corticospinal tract to motoneurons that control finger muscles (Dum and Strick 1991), these connections are not
enough to perform a grasping action as the lesion of the primary motor cortex
(F1) completely disrupts the grasp execution (Fogassi et al. 2001). Therefore, our assumption
that the premotor controller outputs are higher-level signals (in the sense
that they require a subordinate layer to interpret them) is in fact supported
by neurophysiology.
Currently, there is no hand kinematics data synchronized
with F5 firing. Therefore, it is not possible to reliably assert the roles of
different neurons in area F5. Nevertheless, we propose two likely control
structures for area F5. The first one (Figure
7.2) postulates that F5 canonical neurons mediate the
controller development by priming or gating mechanism such that controllers are
differentially associated with different affordances relayed by AIP. F5 neurons
that are recruited by F5 canonical neurons form multiple feedback and
feed-forward controller pairs (motor schemas or modules) and partition the task
space with inter-module competition and F5 canonical guidance.
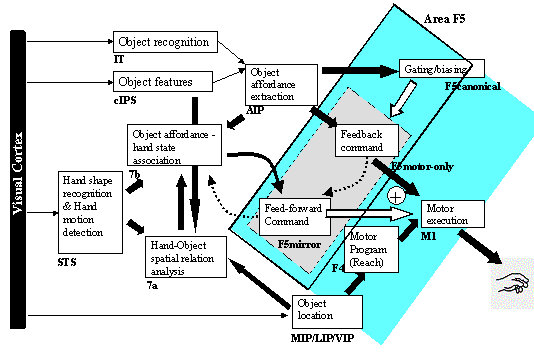
Figure 7.2 One alternative visual
control structure for manipulation is shown within the MNS framework. The
mirror neurons generate feed-forward commands
The complexity of each module depends on the number of
modules that share the task. If the system to be controlled is complex, it is
beneficial to have modularity, as a better overall control performance can be
achieved with controllers specialized for each separate task than with a single
super-complex controller for all the tasks. Wolpert and Kawato (1998; Haruno et al.
2001)
proposed the multiple paired forward and inverse models for this kind of
control scheme. Figure 7.2 expands the MNS model in accordance to this view.
Note that the F5 neurons are now, split into three: canonical, mirror and
motor-only. The motor-only neurons send transient feedback commands to correct
ongoing grasping movements while the F5 mirror neurons provide the motor
command to achieve the final hand configuration or intermediate configurations
as the subgoals of a grasping task. In the second alternative control structure
(Figure 7.3), feed-forward command is generated by canonical
neurons, and mirror neurons are implicated in feedback control of grasping.
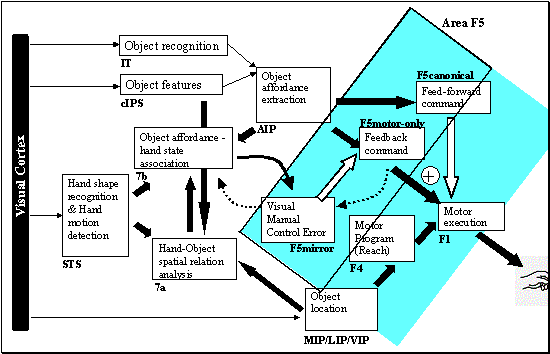
Figure 7.3 Another
alternative visual control structure for manipulation is shown within the MNS
framework (compare with Figure
7.2). The mirror neurons generate feedback commands
In both alternatives, the visual control task is to
1.
Compute a visually defined error based on the current
state and the desired state
2.
Generate a command to reduce the error
From a developmental point of view, the questions one might
ask is what the error and the desired state for the newborn are. Can we assume
that from birth the circuits that compute visual error (e.g. distance to
target) exists in the primate brain? We argue that the answer is negative and
postulate that an LGM-like circuit trains the manual visual feedback control
circuit. In intuitive terms, the animal learns how a successful grasp looks
like by observing the performance of LGM-like circuit. Assuming the animal
learns to extract desired state in visual terms by observing LGM,
we can study how neural circuits in area F5 may use this signal to function as
a manual visual servo circuit. For example, for the precision pinch schema, the
desired state could be the contact of index and thumb fingers on the object
surface. The manually defined Hand State (Chapter 3) that monitors the relation
of the hand to the object could serve as the desired state. However, in the
general setting, we assume that the desired state is learnt from LGM based
grasps.
Figure
7.4, illustrates the basic manual visual feedback control
circuit we propose for the structure that posit mirror neurons as feed-forward
command units. The canonical neurons act as gating units; they select the
appropriate feedback and feed-forward pairs based on the objects to be grasped.
Based on the F5 neurophysiology, we can suggest that there exists a single
controller module (schema) for different type of grasps (e.g. precision, power
etc.).
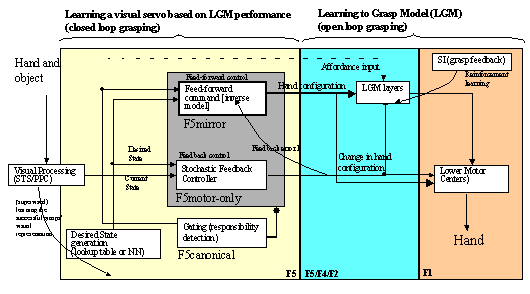
Figure 7.4 The feedback and
feed-forward control view of the F5 grasping circuit, alternative I: F5mirror
neurons learn to generate feed-forward command. The desired state is assumed to
be available and is converted to a correction motor command by F5motor-only
units using stochastic gradient descent. F5canonical neurons gate the
feed-forward and feedback pairs.
The feedback controller receives the desired state and the
current state. The aim of the servo circuit is to generate signals (for
simplicity we will assume the signals determine change in hand configuration),
such that the new state gets closer to the desired state. Note that we don’t
define the state. For MNS model, we defined a common state (hand state of
Chapter 3) which could be used for all the grasps that are accounted, which may
not be a parsimonious choice for the brain. Here each module may learn its own
state. In intuitive terms, it means that each module, for its input can use the
aspects of the action they are in charge of controlling. The key point is that
the state depends on the vision of the hand and the object.
The simplest of such a state is the distance between the hand and the object,
which is a good choice for a pointing task servo circuit, but a bad choice for
a grasping servo circuit since it is clearly not enough to control a hand to
perform a grasp action based on a single scalar parameter. There is psychophysical
evidence that such visual servo systems do exist in man. Ghahramani et al. (1996) in a psychophysical
experiment, limited the visual feedback of finger position at one or two
locations in the workspace, where a
discrepancy was introduced between the actual and visually perceived finger
position. The remapping induced changes in pointing task were largest near the
locus of remapping and decreased away from it. This pattern of pointing
disturbance suggests that visual feedback is used by the reach related
visuomotor circuit of human during pointing.
Figure
7.5, illustrates the basic manual visual feedback control
circuit we propose for the structure that posits mirror neuron involvement in
feedback control. The outputs of mirror neurons are converted to motor commands
by F5motor-only neurons. The feed-forward command is generated by canonical
neurons. Note that the output of mirror neurons cannot be interpreted as a
feedback command in a trivial manner. Most mirror neurons increase activity
during the approach phase where the grasp error is decreasing. It can be
argued that such neurons do not affect the overt movement because their targets
in area F1 are inhibited. Another explanation could be that the mirror neuron
activity works via inhibition, however this is unlikely because the
microstimulation of F5 triggers movement. We propose that mirror neurons, at
any given instant, keep an error map based on their preferred action (e.g.
precision grasp) and visual stimuli.
Thus, the mirror neurons provide the feedback error
(for their preferred actions), on which a feedback command can be generated. It
is up to some other neuron population (e.g. F5 motor-only neurons) to produce a
corrective command based on the population of mirror outputs relevant for the
object to be manipulated.
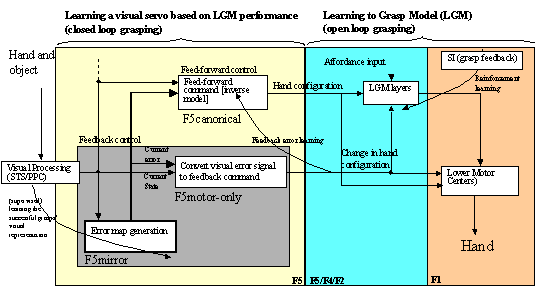
Figure 7.5. The
feedback and feed-forward control view of the F5 grasp circuit, alternative II:
F5mirror neurons learn to compute the error. The error is then converted to a
correction motor command by F5motor-only units. F5canonical generates the
feed-forward command signal.
We based our choice on the following observation. If F5
mirror neurons were lesioned in ‘alternative I’ (the visual servo circuit that
employs mirror neurons as feed-forward elements -Figure 7.4), the model would fall back to feedback only mode and
could perform the action but with the lack of synergy of having the target
configuration (the feed-forward signal). Analogously, it has been shown that
inactivation of F5 mirror neurons in the monkey does not abolish the grasp but
merely slows it. On the other hand, when F5 canonical neurons are inactivated
the grasps that require precision cannot be executed. This agrees with
‘alternative I’ that F5 canonical neurons select the modules to be used in the
grasping tasks where precision is required (so that a feedback control is
necessary). Therefore, we favor F5 control structure that assigns the mirror
neurons the role of feed-forward motor command generation(Figure 7.4).
In the next section, we propose a biologically realistic
visual feedback circuit that can work as an autonomously visual servo circuit
for reaching and grasping. It must be noted that, the computational
background for this task is well established in robotics, although we do not
use it here because of biological implausibility. The required change in
controller output (change in hand configuration) in order to get closer to the
desired state can be achieved using resolved motion rate control techniques
from robotics (Klein et al. 1995;
Whitney 1969).
The computation requires the determination of an input dependent matrix to
convert the (desired state-current state) vector direction to the correct
gradient to be followed by the control output. Once the module learns this
relation, the hand configuration changes for the precise positioning of fingers
becomes possible. The visual servo circuit that will be presented follows the
gradient stochastically. However, stochastic or deterministic, the gradient
following requires constant monitoring of the hand and object to
calculate the instant configuration changes.
It can be beneficial to determine the target configuration
with one-shot computation (feed-forward control). Adopting the
‘alternative I’ (discussed in section 7.2.4), we involve mirror neurons in performing the
feed-forward control function; that is given an object and hand in action the
mirror neurons report the hand configuration required to achieve a grasping
configuration. Once the mirror system has learned an action the animal can act
faster, more accurate and in a more robust-to-perturbation fashion using both
feed-forward and feedback control outputs.
The feed-forward controllers are trained by observing the
successful grasps performed by the feedback controllers. The Reach and Grasp
schema of Chapter 3 used inverse kinematics techniques for implementing the
feedback controller. There the desired states were defined differently for each
grasp type. The desired state information was defined algorithmically. With the
LGM, we are one step ahead. While LGM learns and performs exploratory grasp
plans, the feedback controller(s) can learn the desired states (successful
grasp plans, even if rare) and the relation of input (visual) states to the
output (motor) commands. Then, the learning in the feed-forward controllers can
be accomplished in a biologically plausible way using feedback error learning (Gomi and Kawato 1993) since the error is readily
available as the output of the corresponding feedback controller. One
alternative to feedback learning is using ‘distal learning’ (Jordan and Rumelhart
1992)
approach where first the forward model is trained and then the inverse model is
trained using the error that is propagated backward through the forward model.
However, distal learning is less biological because of the error back propagation.
This relates our discussion to the multiple paired forward and inverse model
architecture introduced by Wolpert and Kawato (1998; Haruna et al. 2001).
This section presents the visual control circuit
implementation outlined in Figure
7.4. The next section, focuses on the feedback controller
module of Figure
7.4 and present a biologically plausible model in the
sense that it can be implemented by leaky integrator units. We sidestep the
complexity of the 3D arm that we used in earlier chapters by switching to a 2D
arm model, but we require the system to learn the reach component as well as
the grasping component. In Chapter 6, we have seen that learning the
affordance-grasp associations produced selective populations for different
grasps. Here without loss of generality, we limit ourselves to precision pinch
grasps and study the control aspects based on kinematics properties of the
reach and grasp movements.
Specifically we
1.
Present a visual servo circuit using leaky integrators that
can reach and grasp (section 7.3.1)
2.
Propose a memory based feed-forward module and expand (1) into
a feedback and feed-forward control system (section 7.3.4)
3.
Present simulation results demonstrating that (1) is effective
4.
Present simulation results with a dynamics model of the arm
showing that (1) and (2) improves controller performance
The results (3) and (4) are meant to convince the reader
that the proposed visual controller based on leaky integrators (feedback) and
memory-based neural units (feed-forward) is adequate as a visual servo circuit.
The readers interested in the main results concerning the mapping between the
mirror neurons and the controller can skip to section 7.4
where we compare feed-forward units activity with real mirror neuron
recordings.
For a feedback controller system, a desired behavior or an
error signal showing the deviation from the desired behavior is required. For
the grasping the desired behavior, is grasping the object. In this model, we
assume that the visual error signal is available for defining the how close the
hand is to its final grasping configuration. A neural circuit can learn to
generate the error signals based on the observed successful grasp examples.
However, we do not model the learning of error signal generation and
concentrate on how the error signal can be used to perform feedback based
grasps.
From a computational point of view, the visual error signal
has to be converted into motor error to deterministically control the behavior
of the system. In this model, we take a stochastic gradient approach where the
system determines its control output based on the commands it has sent in the
previous time steps. One of the goals of the model is to give a simple but
autonomous neural circuit that is open to neural implementation. Therefore, we
avoid computations requiring programming constructs such as if-then, but
instead use differential equations to describe the system. Figure 7.6 shows the schema level view of the feedback
controller. The visual processing encapsulates the process of extracting a
visual error based on the vision of the hand and the object. Lower Motor
Centers encapsulates the functionality involved in transforming the motor
signal sent by the feedback controller into neural signals sent to muscles. In
later sections, we augment and detail the circuit to include the feed-forward
modulel.
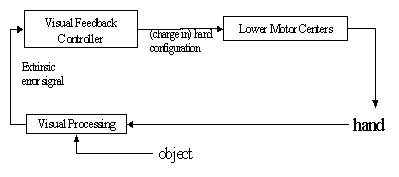
Figure 7.6 The schema level view of the
feedback controller. The visual processing encapsulates the process of
extracting an error based on the vision of the hand and the object. Lower Motor
Centers encapsulates the functionality involved in transforming the motor
signal into actual commands sent to muscles
The feedback controller sees outside world through its input
(the extrinsic error signal) and affects the environment through its output
(the change in behavior) signal. For the task of grasping, we define the output
as the change of joint angles in the arm-hand.
The error signal is taken as the distance of the fingers
used in grasping to the target location on the object. In general, an object
affords multiple grasps. However, the multiple affordances are not a concern
here since Chapter 5 showed how a menu of affordances can be formed and
selected (see also Fagg and Arbib
1998 for prefrontal influences on grasp selection) based on the object
affordances. The hand/arm model is a planar one and comprised of three links, a
thumb with 1DOF an index finger with 2DOF. We only consider precision type
grasp with varying aperture sizes,
which is defined by two points on the object as targets for the fingertips (see
Figure 7.8). The error signal
is then, a four-dimensional (two planar coordinates) and the output is a
five-dimensional (five joint angle changes) vector. The computational elements
we use are biologically realistic (e.g. integration, summation and shunting).
We use leaky integrators as neural units. The representation we use is rate
coding. The output of a leaky integrator unit defines single parameter in
contrast to the population coding we used before. However, we can use single
units without loss of generality because in theory we can expand a unit’s
activity to a population activity and apply the single unit equations to a
population with proper weight coefficients. Figure
7.7 presents the detailed circuitry of the feedback
controller module. The figure represents the differential equations and the
operations in a schematic form. The output of the feedback controller is the
change in joint angles (Dq), and the error signal that
drives the circuit is the sum of the distances of the fingers to their targets
(e(t)). The working principle is based on stochastic gradient descent.
The network tries a random move; if the move was efficient in reducing the
error then it is more likely to make a similar move. If it was a bad move, the
movement is backed up. Although the stochastic gradient method is
algorithmically very simple, it requires some care for implementing it as a set
of differential equations. The merit in implementing an algorithm in terms of
leaky integrators is to show that the computation can be implemented in the
brain. Once this is shown, the algorithm can be encapsulated and used as a
computational block (schema) in designing other brain circuits much like the
winner-take-all circuit (see Arbib 1989, chapter
4.4 for a leaky integrator implementation and mathematical analysis).
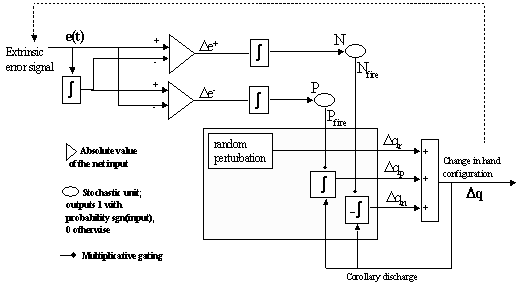
Figure 7.7 The
leaky integrator implementation of the feedback circuit that solves the inverse
kinematics problem for precision grasping. See text for the explanation
The key stochastic elements of the circuit are the firing of
the units P and N (random perturbation can be considered
as a background noise). The units P and N are leaky integrators
where P follows the value of De-(t),
whereas N follows De+(t). The
variables De-(t)
and De+(t)
keep track of the change in error. If the recent commands increased the error De+(t)
charges up, whereas if the recent commands decreased the error De-(t)
charges up.
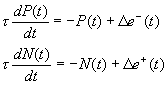
Both parameters De-(t)
and De+(t)
are non-negative and have zero resting potential level. The change in
error is monitored with auxiliary parameter edelayed(t)
which follows the error with a lag determined by the time constant k.
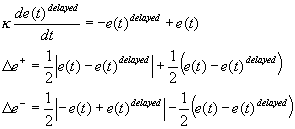
At any instant, Pfire(t)
and Nfire(t) are either 0 or 1 corresponding to the firing of
the units P and N respectively. The probability of each unit’s
firing is determined as the following.
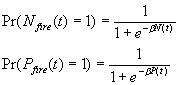
b is the steepness
parameter determining how quick the circuit is likely to respond to a change in
error. This parameter is not critical; however, we empirically found that
setting it to a high value (~100) results in fast convergence. One noticeable
fact is that, unlike many stochastic units (Hertz et al. 1991), the probability is always
greater than 0.5. The output of P and N units gate the three
channels Dqr,
Dqp,
Dqn
that sum up to give the net Dq. These are defined
as:
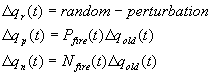
The Dqold keeps
a history of the net output of the controller (Dq) with a time
constant of V.
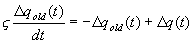
The net output is simply given by
the summation of three sources. Note however that Dqn is
non-negative and therefore its effect is reflected with a negation.
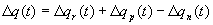
The net output intuitively
interpreted as the following. If both P and N fired at the same
time, claiming that the error both increased and decreased, the net output is
mainly determined randomly (by Dqr). If N
fired but P did not fire, meaning that it is likely that the error was
increasing due to recent commands, then we try to undo the last commands (-Dqn).
On the other hand, if the error was decreasing (P fired but N did
not fire) then we try to repeat what we did before. The final case is when
neither N nor P fires, which results in a random decision (Dqr).
We implemented the proposed circuit using Matlab. For this
section, the arm was implemented as a kinematics chain without dynamics. The
reason was we wanted to avoid the coupling of the dynamics of the arm with the
feedback circuit’s internal dynamics. However, in later sections we introduce dynamics
as well. For solving the differential equations that define the circuit, we
used Euler integration with time step 0.01. The command is sent to (and in the
kinematics case, applied immediately to the arm’s configuration) at each 0.1
time units.
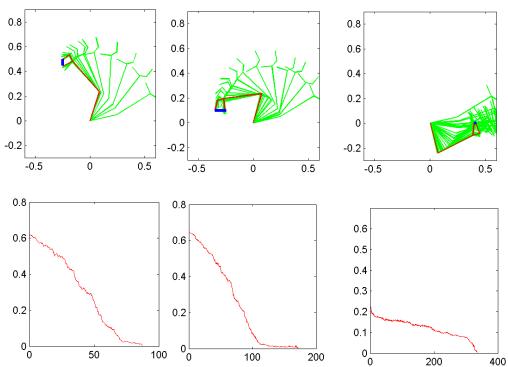
Figure 7.8 Three
grasping tasks executed by the feedback circuit proposed shown on the upper
half of the figure. The change of arm/hand configuration during the execution
is illustrated by snapshots of the arm/hand. Each hand figure is accompanied
(lower half) by the error plot. The grasp execution is stopped (success) when
the sum of finger distances to their target was less than 2mms.
The Figure
7.8 show some example grasps performed together with the
error plot. Each tick in the time axis of the error corresponds to 0.1 time
units. For example, the leftmost grasp required less than 100 commands while
the rightmost harder grasp required more than 300 commands. The rightmost
grasp is harder because on the way to the target there are local minima to be
overcome.
From a control theory point of view, it is important to ask
the question how the lower motor centers will work with the feedback
circuit we proposed. To answer this question we first need to implement a
dynamics model of the arm and then design a motor controller, which tracks a
given trajectory. Simplest such a controller is a PD controller with suitable
gains. Note that we implicitly assume that PD controller is encapsulated in the
lower level grasp schema that we have postulated to receive the output of the
visual servo output signals. Figure
7.9 shows the control system we implemented. For the arm,
we assumed that hand’s contribution is negligible and set the upper limb length
as 0.25 meters and weight as 4kgs, and the distal limb length as 0.35 meters
and weights as 3kgs. Assuming cylindrical links and homogeneous mass with no gravity
the 2D arm dynamics can be given by (Sciavicco and Siciliano
2000):
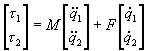
Where M is the inertia matrix
and F is the matrix of Coriolis and centrifugal forces. Note that we
don’t include a gravity term since we assumed no gravity.q1 and q2
are the joint angles of the shoulder and the elbow respectively. t1
and t2
denotes the corresponding torques. Single and double dot notation refers to
usual first and second time derivatives. The inertia matrix and the matrix of
Coriolis and centrifugal forces are given by:
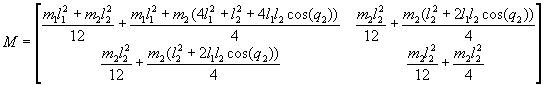
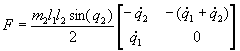
The PD controller loop has to be faster than the visual
feedback controller loop. Otherwise, the PD controller will not be able to
track the desired joint angles specified by the visual feedback controller. In
our simulations, we used 250 Hz. for the PD controller loop and 10 Hz for the
visual feedback servo.
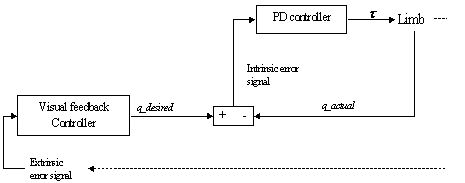
Figure 7.9.The
Visual feedback circuit generating desired trajectories for the ‘lower level
motor centre’ (implemented as a PD controller)
The cycle rate of the PD controller becomes more important
when tracking high-speed trajectories. The PD gains can be selected to reduce
tracking error, but the cycle rate of the controller brings a limitation on
that also. In this simulation, the position gain was chosen as 30 and the
velocity gain was chosen as 10. In Figure
7.9, this speed effect can be observed. The bottom half
of the Figure
7.10 shows the tracking error and the followed path when
the action took 2 seconds whereas the upper half shows similar graphs when the
action took 0.5 seconds. When the action was slow, the combination of visual
feedback circuit and the lower motor centers can work well (Figure 7.10, lower half). However, for higher speeds the circuit
is not very effective for precise guiding of the hand (Figure 7.10, upper half).
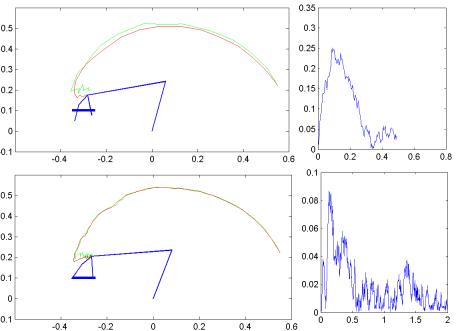
Figure 7.10 The
slow (2 seconds) (lower half) and fast (0.5 seconds) (upper half) performance
of the ‘visual feedback servo’ + ‘PD controller’ system is shown. The right
hand side graphs show the tracking error (of the wrist) versus time. In the
slow case, the object can be grasped but in the fast case, it is missed
It is desirable to generate desired trajectories in ahead
without requiring a correction afterwards. In our simple visual feedback
controller the system has to make a move; if it is not good it has to undo it
and perform another move and so on. The desired trajectory produced in this way
is not very smooth and would suffer from the feedback delays in a real robotics
or biological system. The system we had could work pretty well (Figure 7.10) because we assumed the feedback signal has no
delays. Having only the feedback signal as the corrective mechanism has severe
consequence on the controller performance when there is delay in feedback loop.
To overcome this we can use a feed-forward controller and issue the right
command without requiring the feedback signal. The perturbations that cannot be
accounted for (external perturbations for example) have to be still taken care
of by the feedback controller. In the next section we augment the visual
feedback servo circuit with a feed-forward module addressing one of the
important elements of the visual control of grasping circuit we proposed (Figure 7.4) and show how the feed-forward units can behave as
mirror neurons.
In this section, we augment the feedback controller of the
previous section with a feed-forward module. The feed-forward module is formed
by neural units that are selective to visual grasping errors. During any
grasping action a number units activate based on their match on the object-hand
relation. The one that fires maximally specifies the current feed-forward
command. We define the feed-forward command in the visual control framework and
introduce the mirror neurons as candidate for feed-forward command formation.
We present our design of the circuit and present simulations
performed using the planar arm/hand we used in the previous section.
Importantly we take a bold step and try to establish a link between F5 mirror
neurons by comparing the activity of real F5 neurons with the simulated
feed-forward units.
7.3.4.1
Mirror neurons, inverting actions ?
The natural way to construct a feed-forward controller is to
invert the controlled plant. If the plant is characterized by x=f(u),
where x is the behavior of the system and u is the command used
to manipulate the plant, then if we can compute
f-1(x) we will have a perfect feed-forward controller,
because the analytic relation u=f-1(x) unambiguously
tells us what command u is required to achieve a desired
behavior. Now let us motivate why F5 mirror neurons can be involved in such an
inverse computation. We know that F5 mirror neurons that are involved in
encoding motor plans are activated by the observation of similar actions (Gallese et al. 1996;
Rizzolatti et al. 1996a).
An inverse computation in a visuomotor task is intuitively defined by: ‘given a
visually defined desired behavior what is the required motor representation?’
Thus, if the mirror neuron activity represents some part of a motor plan then
we can view them as elements of a feed-forward control system. Assuming a
visual feedback controller exist as we described in the previous section, then
even without F5 mirror neurons we can expect to have visual control of grasping
with limited degradation (especially in slow movements the delays in feedback
becomes less severe). Indeed, in the study of (Fogassi et al. 2001) even though the grasping
movements were not abolished when F5 mirror neurons were inactivated, there was
a slowing in the grasping movements but the hand reaching and preshaping
were intact. Although we argue in favor of F5 mirror region’s being an inverse
model we need to emphasize that a single mirror neuron itself cannot be an
inverse model for a visual control task since the single neuron activity of a
neuron is much too variable to encode a precise action. The activity of mirror
neurons can be strictly selective for the type of the grip used (e.g. precision
or power grasp) or broad (Gallese et al. 1996;
Rizzolatti et al. 1996a).
If the mirror neurons encode motor plans, the plan must be represented in a
distributed fashion.
Now we turn back to our visual controller and specify the
possible input output parameters for the feed-forward controller, which we advocated
F5 mirror neurons for. In the simple grasping world we defined, a feed-forward
command would specify the (change of) arm configuration (the joint angles)
given the visual information about the object and the hand. The nature of the
visual information deserves some comment. The intrinsic grasp related object
properties (affordance) are relayed to area F5 via AIP, which is reciprocally
connected with F5 (Matelli 1986). The object location
information can be relayed to area F5 via other parietal regions such as VIP (Duhamel et al. 1998;
Colby et al. 1993a)
or premotor regions such as F4 (Fogassi et al. 1992;
Fogassi et al. 1996).
For grasping it is likely that area F4 and F5 work together with F4 being
involved in the reach component and F5 in grasp component. Also there is
evidence that F2 may be involved in controlling wrist rotations (Raos et al. 1998). However, in our simple grasp
world we assume without loss of generality, that our inverse model specifies
the full arm configuration without explicitly making a task division over
mentioned possible premotor regions.
7.3.4.2
Inverse kinematics and requirement for local representations
From a computational point of view the task of feed-forward
command is simply stated as finding the inverse mapping of f(u),
which is the forward function describing how the plant behaves with given
command u. Our case f(.) function is referred as
the forward kinematics mapping and f-1 is
called the inverse kinematics mapping. Mathematically speaking f-1(.)
may not exists because f(.) need not be one-to-one, that is f(u1)=f(u2)
does not necessarily imply that u1=u2. In
this case, the manipulator (e.g. arm/hand) is called redundant. Even our
simple arm/hand is redundant. To see this, simply note that the wrist can move
without breaking the finger contacts on the object during a two-finger pinch.
In the literature there are many techniques to solve the inverse kinematics
problem (Flash and Sejnowski
2001; Klein et al. 1995; Whitney 1969). In a redundant system, the
inverse kinematics problem can be solved by introducing an extra optimization
criterion such as minimum energy and shortest distance. The commonly used resolved
motion rate control (Whitney 1969) methods rely on the matrix
called the Jacobian of the forward kinematics which is composed of the partial
derivatives 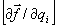 of the forward
mapping function. The Jacobian transpose, pseudo-inverse of the Jacobian (with
null space optimisation), and the extended Jacobian (Klein et al. 1995) methods are widely used in
robotics and computer graphics for mapping the extrinsic coordinates into joint
angles (Sciavicco and Siciliano
2000).
However, we are interested in solving the task using neuron like units rather
than pure engineering techniques. There is also a plentiful of robotics neural
network literature on learning inverse kinematics functions (Tang and Wang 2001;
Oyama et al. 2001a; D'Souza et al. 2001; Driscoll 2000). The key point in solving the
inverse kinematics for a redundant manipulator is to employ a modular neural
network (Oyama et al. 2001b) or to use locally specialized
learning methods such as Locally Weighted Regression (LWR) (Atkeson and Schaal
1995; Schaal et al. 2002).
The reason is that usually the inverse kinematics function is not convex, thus
the average of two solution points may not be a valid solution point. A
homogeneous non-local neural network (e.g. a back-propagation feed-forward
neural network) averages over two solution points when queried with a
non-trained point (unless a negative data point in the region of query is
manually added to the training set to disable the ‘wrong’ averaging).
The simplest locally weighted algorithm is the memory-based LWR (Atkeson 1992; Atkeson
1989; Atkeson and Schaal 1995).
of the forward
mapping function. The Jacobian transpose, pseudo-inverse of the Jacobian (with
null space optimisation), and the extended Jacobian (Klein et al. 1995) methods are widely used in
robotics and computer graphics for mapping the extrinsic coordinates into joint
angles (Sciavicco and Siciliano
2000).
However, we are interested in solving the task using neuron like units rather
than pure engineering techniques. There is also a plentiful of robotics neural
network literature on learning inverse kinematics functions (Tang and Wang 2001;
Oyama et al. 2001a; D'Souza et al. 2001; Driscoll 2000). The key point in solving the
inverse kinematics for a redundant manipulator is to employ a modular neural
network (Oyama et al. 2001b) or to use locally specialized
learning methods such as Locally Weighted Regression (LWR) (Atkeson and Schaal
1995; Schaal et al. 2002).
The reason is that usually the inverse kinematics function is not convex, thus
the average of two solution points may not be a valid solution point. A
homogeneous non-local neural network (e.g. a back-propagation feed-forward
neural network) averages over two solution points when queried with a
non-trained point (unless a negative data point in the region of query is
manually added to the training set to disable the ‘wrong’ averaging).
The simplest locally weighted algorithm is the memory-based LWR (Atkeson 1992; Atkeson
1989; Atkeson and Schaal 1995).
The feed-forward module for the visual servo circuit uses
the memory-based learning with nearest neighbour (Atkeson and Schaal
1995).
The reach model of Rosenbaum et al. (1995) showed that summing over
postures is not a feasible strategy (Rosenbaum et al. 1999). Thus, when querying a point
we do not apply a weighted sum as in LWR but simply return the nearest match
with a winner–take-all circuit. This ensures that we never have a wrong answer
to a query. In their planar kinematics grasp simulator Rosenbaum et al. (1999) used the same approach with
the additional explicit constraint satisfaction criterion.
The learning takes place in
this fashion: when the hand is performing a grasp action servo-ed by the
feedback controller new F5 feed-forward units are allocated when the hand
configuration is not similar to any configuration encountered before. The
instantiation of the configuration is based on joint angles (and visual gating
signals). As a new configuration is stored its corresponding visual
representation is stored along with it.
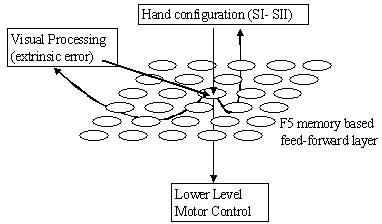
Figure 7.11 The F5
mirror neurons viewed as the memory based feed-forward controller. The arrows
below the sheet of neurons indicate outputs while the arrows coming above the
sheet indicate inputs
A F5 memory unit is activated to the extent that the hand
configuration and the visual error fall into its receptive field.
The activity signals what change must be done given the extrinsic error and the
current hand configuration establishing an inverse kinematics circuit. In this
model, the lower level applies a winner-take-all process on the activity of F5
feed-forward neurons. The result is then used for movement execution (Figure 7.11).
To our knowledge, there is no experimental data
as to how a grasp plan is represented in premotor cortex. Therefore, we are not
claiming that the brain uses exactly these mechanisms; but rather we are
proposing a distributed feed-forward module that can supply a
feed-forward signal complementing the feedback signal. However, when we show
later in this chapter that it is possible to observe unit activities that are
similar to mirror neuron activities, we suggest that the mirror neurons may be
involved in encoding visually defined grasp errors. It should be understood
that our modeling in this chapter is not intended to be a proof that the
premotor cortex works as we modeled, but rather an important message to
experimentalists that quantitative experiments are necessary to pin down the
mechanism of grasp planning and execution.
Now let us look into the details of our representation for
F5 mirror neurons. There are two kinds of inputs to F5 mirror units: (1) the
somatosensory cortex input that signals the configuration of the hand/arm. We
capture this input as a vector of joint angles of the arm/hand: s=[q1,q2,q3,q4,q5]T
(shoulder angle, elbow angle, thumb angle, index finger angle, index finger
second metacarpal angle), and (2) inputs from the visual centers described as
the following. The visual input to feed-forward units is a five dimensional
vector x=[dx1,dy1,dx2,dy2,gA].
The first four components are the parameters are the errors signaled by
the feedback loop, namely the distances between fingertips and their contact
targets on the object. The last component represents the affordance of the
object. In this model, we use only the size of the object as the affordance.
The parameter g
controls the relative importance of the error and the object affordance input.
Note that gA parameter plays the role of a
soft gating network. As we advertised earlier, F5 canonical neurons may
implement an explicit gating network based on visual object properties (Murata et al. 1997a) for biasing F5 mirror
units. In that case, only the
feedback error signals would suffice, which is in full accordance with the
learning structure we offered in Figure
7.4. However, for the sake of implementation we embed the
gating mechanism into the error signal. It must be emphasized that x
is purely defined within an extrinsic space. Thus, it can be applied to
self-action as well as the actions of others. However when learning (storing of hand configurations) the recruitment of new units to store a
configuration is based only on intrinsic parameters (s vector).
The circuit continually monitors the arm it controls (i.e. online learning); if
the maximum activation based on s input of the units is below a
threshold a new unit is recruited. The hand configuration activity si of
unit i, is computed simply as the length of the vector between s
and si (|s-si|) where s is the current hand configuration
and si is the stored configuration i. Figure 7.12 shows the acquired configurations during the
simulations of this chapter for six objects. In a general system for
satisfactory performance many more units are required (Rosenbaum et al. 1995; Rosenbaum et al. 1999;
Rosenbaum et al. 2001).
Figure 7.12 The arm
configurations that were acquired during 6 object grasping actions are shown. Each of the superimposed configurations is represented by a unit in the
feed-forward layer
We mentioned (1) configuration dependent
activation and (2) vision based activation. Now we need to explain the
mechanism of inverse mapping, that is, how the given visual information is
mapped to a motor plan. At any instant, during a grasp action, the vision based
activity and the hand configuration based activity lights up some candidate
configurations. The task is to pick one of the units as the best candidate and
return its stored hand configuration as the next target (as the joint angles).
In a general framework, a sophisticated way of combining activated units would
be employed as in LWR methods. We speculate that the brain circuits must have a
reinforcement type of learning circuit to learn to pick a chain of hand
configurations that will satisfy extrinsic constraints (obstacle avoidance) and
intrinsic constraints (such as minimum energy). However for simplicity we offer
a heuristic for selecting a configuration that will enable the system to
generate reasonable trajectories so that we can test the system’s control
performance and look at the properties of the units that are acquired. Our
heuristic is to pick the unit that maximizes the activity function: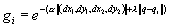 where a and l are constants controlling the relative contributions of the intrinsic and
extrinsic signals. Here there is a subtle issue: the minimum of g may not be a
right plan because the error vector [dx1,dy1,dx2,dy2]
is relative to the given object. To circumvent this problem there are two
possible mechanisms that can be applied: (1) having a gating mechanism which
biases the correct subspace of the error space based on the object affordance
(this is the task we offered for F5 canonical neurons), (2) having a forward
model of the arm/hand kinematics that predicts the error vector if the unit
were selected and use this predicted error [dx1,dy1,dx2,dy2]’
in the computation of
where a and l are constants controlling the relative contributions of the intrinsic and
extrinsic signals. Here there is a subtle issue: the minimum of g may not be a
right plan because the error vector [dx1,dy1,dx2,dy2]
is relative to the given object. To circumvent this problem there are two
possible mechanisms that can be applied: (1) having a gating mechanism which
biases the correct subspace of the error space based on the object affordance
(this is the task we offered for F5 canonical neurons), (2) having a forward
model of the arm/hand kinematics that predicts the error vector if the unit
were selected and use this predicted error [dx1,dy1,dx2,dy2]’
in the computation of 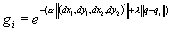 . It is suggested that both gating and forward model strategy
is employed in the human brain from cerebellar motor prediction to social
prediction (Miall and Wolpert 1996; Wolpert et al. 1998;
Wolpert et al. 2001). For our simulations the winning unit is
selected using the latter approach (we use a non-neural forward model).
. It is suggested that both gating and forward model strategy
is employed in the human brain from cerebellar motor prediction to social
prediction (Miall and Wolpert 1996; Wolpert et al. 1998;
Wolpert et al. 2001). For our simulations the winning unit is
selected using the latter approach (we use a non-neural forward model).
Although it is a standard result that use of feed-forward
signals improves performance we need to first show that the distributed
feed-forward system we proposed does not degrade but improves the performance
as expected.
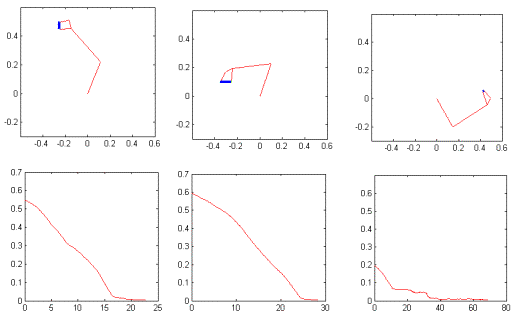
Figure 7.13 The
trajectory generation with feedback and feed-forward control is illustrated for
comparison with Figure
7.8 (feedback-only system). In the lower panel the error graphs
are plotted as error versus iteration. The error is the sum of squared
distances of the fingertips to their targets. The rightmost object was not
grasped in the training (a novel object/location). Thus the system could not
make use of the feed-forward signal, approximately after iteration 25 and
switched to feedback only mode, resulting in slower positioning of the fingers
on the target locations
For comparison purposes, we tested the speed of the desired
trajectory generation. Figure
7.13 shows the three grasps that are performed using the
feedback and a trained feed-forward controller. The discrepancy of the errors
at time=0 for Figure
7.13 and Figure
7.8 is due to the initial finger configurations of the
hand (but the arm started from exactly the same configuration). In fact, the
initial error for the middle grasp is larger in Figure 7.13.
The feed-forward control introduction to the system reduced
the time required to output a desired trajectory command by about four times.
This means that when we actually connect the system to the lower level motor
servo we can achieve 4 times frequency that of a feedback-only system could
achieve. This effectively gives a higher range of the PD gains for the lower
level motor servo.
The system learned online
while it was performing grasps using the feedback and feed-forward model
learned so far. The leftmost two grasp objects in Figure 7.13 were included in the training; thus, the error curve
goes down very fast since the feed-forward command can take care of most of the
task (as it acquired an inverse map during training). However, the rightmost
object was presented the first time to the model. The feed-forward module could
make use of the earlier experiences until step 25, only partially between 25,
and 40. After that point, the feedback servo worked alone. Thus, the generation
of the trajectory was slower. We did not use the lower level motor servo to be
able to make a fair comparison with Figure
7.7 of feedback-only system. Figure 7.14 shows the complete systems’, i.e. the feed-forward,
feedback and lower level feedback motor servo performance all working together.
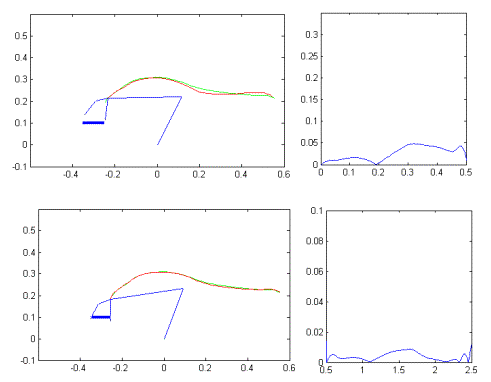
Figure 7.14 The feedback, feed-forward and lower level motor servo and the dynamic arm
was simulated all together. Upper half: The grasp lasted 0.5 seconds. Lower
half: the grasp lasted 2 seconds. The fast movement error reduced with a factor
of 6 while the slow movement reduced with a factor of 10 in terms (compare with
Figure 7.10)
The plots in Figure
7.14 use the same scales as Figure 7.10, for direct comparison. A high degree of improvement
is observed as expected. The slower motion trajectory can be followed with 1/10
times and the fast motion with 1/6 times the error made with only feedback
controller. The reason for the poor performance of the feedback only control
case (Figure
7.10) is that the generated desired trajectories are not
very smooth, which causes the PD controller to overshoot and follow the
trajectory harder. In contrast when using the feed-forward controller, the
generated trajectories are smooth. Note that both PD controllers of the lower
level motor servo used the same PD gains (30 for position and 10 for derivative
gain). The values were manually optimized for the feedback-only system and it
was not possible to increase the gains further to achieve better tracking. In Figure 7.14 even with the feed-forward controller it looks like
the system did not achieve a satisfactory grasp. However, note that we required
the simulator to complete the grasp in the allocated time; the system completes
the grasp after the allocated time with a negligible increase in the error (not
shown).
We mentioned earlier that having memory-based feed-forward
controller may allow explicit planning using the controller representation.
Here we demonstrate this fact by a simulation. An obstacle in the workspace for
example, can be encoded as simple inhibition over the feed-forward units that
encode hand configurations that would result in a collision with the obstacle. Figure 7.16 shows two grasp examples in the top row where the
effect of the obstacle is zero (the obstacle is drawn for reference). In the
lower level the obstacle inhibits the cost function associated with the units
that would bring the hand in the obstacle. Considering the limited range of
motions available to move the arm from its initial position to the target, we
can suggest that the representation we proposed allows generation of
non-trivial trajectories that can be automatically acquired (in contrast to set
by heuristics) once the system is equipped with a learning mechanism that can
do the planning (e.g. reinforcement learning).
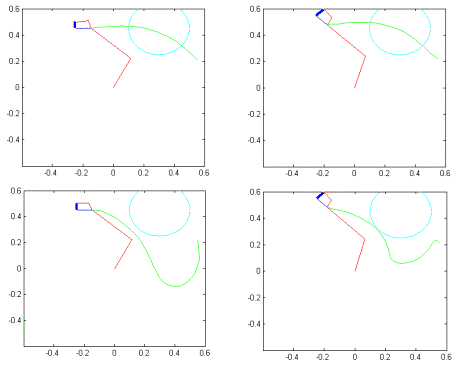
Figure 7.15 The top row demonstrates two
trajectory-planning examples for grasping without obstacle. The bottom row
demonstrates how new trajectories are formed by introduction of an obstacle as
a local inhibition on the feed-forward controller units
In this section, we focus on the unit level activities of
the feed-forward controller and introduce a method to compare the
electrophysiological recordings from monkey mirror neurons with our
feed-forward units activities.
First we look at the population level activity of the
feed-forward (F5 mirror neurons) that we introduced in section 7.3.4.3
during various grasp actions. Figure
7.16 shows the unit activities of four grasps as area
level plots. Each plot consists of 157 neurons acquired during the learning
phase (the rows). The columns represent the time (note that in general the
grasp actions take different time steps for different grasps). The left edge of
each graph is aligned with the start of the grasp actions. The right edge is
aligned with the completion of the grasps. We can see that different units are
activated at different times depending on the grasp action. It must be
emphasized that this map is based on only visual information and mainly
dominated by the error patterns that occur during a grasp action. It is likely
to have similar errors during different grasps unless the map is modulated by
object affordances. We used the object size as a soft modulator by including it
in the error computation. How much object related information is actually
encoded in such a map in the primate is a topic of future research for
experimentalists. Our predictions and experiment suggestions for object
specificity is presented in Chapter 6. Our goal here is to show that a visual
error-based activation of units can lead to similar firing patterns of
mirror neurons.
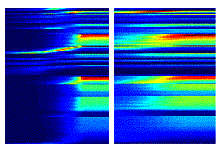
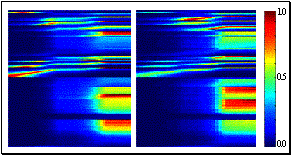
Figure 7.16 The
feed-forward unit activations for four grasp observations shown as unit versus
time. Each graph consists of 157 neurons acquired during the learning phase
(the rows). The columns represent the time
The activity of a feed-forward unit corresponds to average
firing rate of a real neuron. We can map the average firing rates during an
action (observation activity only) to actual firings (neuron spikes) using a
Poisson distribution model of a neuron. The Poisson distribution is the
extension of the binomial distribution to the continuous case. Under the Poisson
encoding model the probability of a neuron generating r spikes
during (t, t+dr) for encoding a parameter x
is given by (Snippe 1996; Sanger
1996; Zemel et al. 1998):
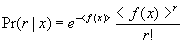
where <> denotes the average firing rate and f()
is the response function (or tuning function) of the neuron which actually
determines the average firing rate activity one computes using a non-spiking
neural circuit model.
Zemel et al. (1998) suggest that a typical tuning
function is proportional to a Gaussian:
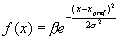
where xpref denotes the input
that best activates the neuron; x is the current input to the
neuron and b
is a proportionality constant and s2 is
the variance determining the receptive field size. We used this form of
activity function for F5 feed-forward controller units. We set b=1 and
empirically determined a single s2 (0.1) value
for all the neurons in our simulations.
The feed-forward units
activation (the average firing
rate) is determined by the activation
function we have defined in
section 7.3.4.3. However, note
that intrinsic contribution is set to zero during an action observation (i.e.
the activity is purely visual during observation). This can be accomplished in
the primate brain by shunting of intrinsic input when the animal is not engaged
in any motor act. Noting that in section 7.3.4.3 we defined x=[dx1,dy1,dx2,dy2,gA]T we can write the probability of a feed-forward units firing based on the
units preferred stimulus xpref and the current
stimulus x as the following.
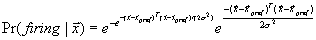
Now we can convert our average
firing rates plotted in Figure
7.16 into spikes and make (qualitative) comparisons with
real mirror neuron data. The mirror neuron data available to us does not allow
comparisons based on kinematics of the observed actions. Therefore, instead we
try to spot feed-forward units that have firing profiles similar to those
available to us.
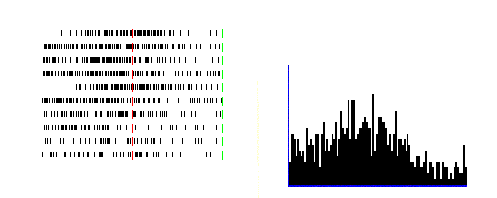
Figure 7.17 A
mirror neuron recorded during a grasp observation. On the left the raster
plots; on the right the histogram. The recording data shown spans 2 seconds. In
addition, the hand start to move approximately at time = 1 second indicated by
the vertical bar at the centre of the raster panel (Rizzolatti and Gallese
2001)
Figure
7.17 shows a real recording from Rizzolatti lab (Rizzolatti and Gallese
2001; Umilta et al. 2001),
which is displayed using the tool we have developed (Oztop 2000). The recording shown spans
two seconds and the end of the rasters are aligned with the experimenter’s
touch of the object. We did not include the holding phase of the recordings for
the comparison because different processes may be involved in holding, which we
did not model. Figure
7.18 (lower panel) shows one of the feed-forward module unit’s
activity that is produced using the Poisson model described above. The
generated spikes are shown as raster plots and histogram for a direct
comparison. The raster plots corresponds to actual trials in the real
experimentation (10 trials), however in the simulation we collected the average
firing rate information via a single trial and run the Poisson spike generation
multiple times (25 runs) The histogram bin width is selected as 20ms for both
experimental and the simulation case. The mirror neuron in Figure 7.17 (same neuron is also shown in Figure 7.18, upper panel) shows an interesting behavior: first,
the activity of neuron rises but before contact with the object, around half
way of the grasp the activity reduces again. This type of behavior is not very
well understood. If this mirror neuron were involved in understanding the
meaning of actions as suggested in the literature (Rizzolatti et al.
2001a; Rizzolatti et al. 2000; Umilta et al. 2001), then one is tempted to ask
the question, why the firing is inhibited prior to contact. To our knowledge,
there is no satisfactory answer to the question and no published explanation of
such mirror activity. Here we demonstrate that such an activity can be
generated as a by-product of the visual servo circuit (the feed-forward module)
that we implemented.
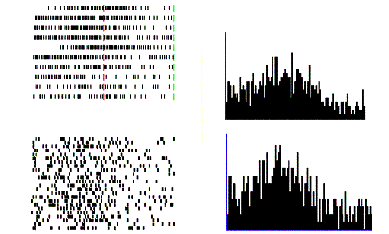
Figure 7.18 Top
row: Real mirror neuron recording during a precision grasp. Bottom row: One of
the feed-forward controller unit’s responses to vision of a grasping action. In
the left panels, each raster row corresponds to a trial (Poisson spike
generation for the model). The right panels show the histograms. The rasters
aligned according to the contact of the hand with the object
We now switch our attention to a more common mirror neuron
that increases its activity as the action observed is progressing towards
completion, which is in support for the mirror neuron involvement in
understanding the meanings of actions (Rizzolatti et al.
2001b).
We can find many units in the feed-forward module, which mimics the activity of
this type of mirror neurons with different rise time profiles.

Figure 7.19. The similarity of a real
neuron and model unit is demonstrated. Left two panels real mirror neuron
rasters and histogram. Right two panels are the model generated rasters and
histogram. A slow increasing activity is observed in both cases
In Figure
7.19, we picked one, which matches a real mirror firing
profile (also shown in the same figure). Note that no parameter fitting was
done; the parameters of the model and the Poisson spike generation process were
the same as in the previous example.
Next, we present an interesting mirror neuron profile, which
we could not replicate without changing the receptive field size parameter (s2).
This neuron was silent as the background activity and stayed silent when the
experimenter started the grasping action (Figure
7.20, left two panels). Before contact, it started firing
vigorously and stopped before holding phase. As the very first neuron we have
presented we suggest that this neuron must be involved in some processes other
than understanding because of the similar reasoning. When we tried to find this
kind of activity in our feed-forward control module at first we could not find
such sharp profile units in any grasp action observation. However, when we
reduced the receptive fields of the units we could get similar activity (Figure 7.20, right two panels) although the timing of such
activity could not be replicated (the length of the silent time after the
burst). However, this could be because we require the grasp to be completed for
the simulator case. The real mirror activity shown is only until the contact of
the hand to the object. Inspecting Figure
7.21 indeed reveals that the final portions of the
grasping action do not change the population activity. Nevertheless, it is
certainly too daring to claim that the mirror neurons use the same error space
we used for the simple grasp world we implemented. However, we would like to
suggest that mirror neurons can be involved some visual error computation for
visual guidance of the hand during grasping.
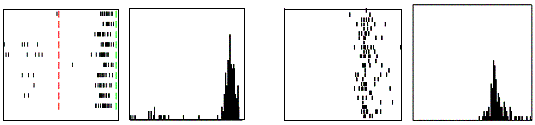
Figure 7.20 Left: a
sharp mirror neuron activity, which could only be replicated with our simulator
by reducing the receptive field. Right: Similar response profile obtained from
one of the feed-forward module units
When we look at the population activity of the feed-forward
units with the smaller receptive field we see that the main characteristics are
preserved, but the tuning of the units become stricter (Figure 7.21). In the general case, each unit may have their own
receptive field sizes, which can adapt with experience. The unit shown in Figure 7.20 is marked with an ellipse in Figure 7.21. The unit was activated strongly for a short period
of time and declined its activity rapidly. A group of other neurons became
active after the decline.
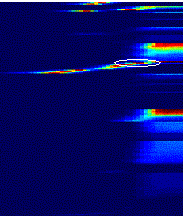
Figure 7.21 The
population activity of feed-forward units with smaller receptive fields. The
feed-forward unit we used to match the real mirror firing profile in Figure 7.20 is marked with an ellipse
The scope for the dissertation was set in Chapter 3 where
(1) the visual feedback control of manual manipulation hypothesis of F5 neurons
and (2) the view that infant grasp learning precedes the mirror neuron
development were introduced, and (3) the assertion that self-grasp observation
trains the mirror neurons during infancy was made. Our study took a schema
based approach to address (1), (2) and (3).
First (3) was modeled in Chapter 3, focusing on the Core
Mirror Circuit. The context for the circuit was provided by the functional
schemas we implemented without intending to address neural localization. This
enabled us to concentrate on the Core Mirror Circuit thoroughly and make
testable predictions.
The view (2) motivated us in developing the Learning to
Grasp Models. Chapter 5 developed a behavioral level model of infant grasp
learning, while Chapter 6 localized the circuit in monkey brain. Both chapters
produced nontrivial predictions that can be experimentally tested.
Finally, Chapter 7 attempted to realize (1) through
simplified but nevertheless adequate (according to the F5 canonical priming
hypothesis) simulation studies. It was shown that units encoding kinematics
based errors, could yield mirror neuron like responses. This is in full
accordance with the suggestion in Chapter 3 that, the degree of conformity of
the hand movement and preshape to the object affordance must be represented as
a distributed neural code. This code in its simplest form is a kinematics error
function which we have elaborated in Chapter 7.
We concluded our modeling tour of action recognition and
visuomotor transformation by handing neurophysiologists an extensive set of
testable predictions and hypotheses, and equipping the modelers who are
interested in understanding the cortical mechanism underlying the action
recognition and visuomotor learning, with an excellent starting guide.
Chapter 3 studied how self-executed grasp actions can adapt
the parietal and premotor connections to shape F5 neurons into mirror neurons.
We used schema approach for the Visual Analysis and Reach and Grasp components
of the Mirror Neuron System (MNS). Therefore, the neurophysiological predictions
pertaining to MNS model could be gathered only from the Core Mirror Circuit,
whose output corresponded to the mirror neurons (see Figure 3.5).
The key to the MNS model was the notion of hand state as encompassing data required
to determine whether the motion and preshape of a moving hand may be
extrapolated to culminate in a grasp appropriate to one of the affordances of
the observed object. A mirror neuron had to fire if the preshaping of the hand
conformed to the grasp type with which the neuron was associated. We emphasized
that the specific decomposition of the hand state into the specific components
that we had used in our
simulation was not the crucial issue. In fact, we suggested that a distributed
neural code that carries information about the movement of the hand toward the
object, the separation of the virtual fingertips and the orientation of
different components of the hand relative to the opposition axis in the object
must be represented in monkey parietal or premotor cortices. The crucial
property of the code we predict is that it would work just as well for
measuring how well another
monkey’s hand is moving to grasp an object as for observing how the monkey’s
own hand is moving to grasp the object. This allows the self-observation of the
monkey to train a system that can be used for observing the actions of others
and recognizing just what those actions are.
Experimental work to date tends to emphasize the actions to
be correlated with the activity of each individual mirror neuron, while paying
little attention to the temporal dynamics of mirror neuron response. Our
simulations make explicit predictions on how a given (hand state trajectory,
affordance) pair will drive the time course of mirror neuron activity. For example,
we have shown that a grasp with an ambiguous prefix (e.g. using precision grip
for a large object) drives the mirror neurons in such a way that the system at
first gives weight to the wrong classification, with only the late stages of
the trajectory sufficing for the incorrect mirror neuron to be vanquished (grasp
resolution).
The grasp resolution prediction is testable and
indeed, suggests a whole class of experiments. The monkey has to be presented
with unusual or ambiguous grasp actions. For example, the experimenter can
grasp a section of banana using precision pinch from its long axis. Then we
would expect to see activity from power grasp related mirror neurons followed
by a decrease of that activity accompanied by an increasing activity from precision
pinch related mirror neurons. Our other simulations lead to different testable
predictions such as the mirror response in case of a spatial perturbation
(showing the monkey a fake grasp where the hand does not really meet the
object) and altered kinematics (perform the grasp with different kinematics
than usual). We have also noted how a discrepancy between hand state trajectory
and object affordance may block or delay the system from classifying the
observed movement.
In summary, we have conducted a range of simulation
experiments – on grasp resolution, spatial perturbation, altered kinematics,
temporal effects of explicit affordance coding, and analysis of compatibility
of the hand state to object affordance – which demonstrate that MNS model is not
only valuable in providing an implemented high-level view of the logic of the
mirror system, but also provides interesting predictions that are ripe for
neurophysiological testing, as well as suggesting new questions when designing
experiments on the mirror system.
Chapter 7 investigated the manual visual feedback
hypothesis asserted in Chapter 3 through a simplified hand/arm system. We
concentrated on a single grasp type. However, this does not weaken the impact
of the analyses we conducted since Chapter 6 demonstrated, through explicit
simulations, that grasp learning gave raise to emergent neural properties
comparable to F5 canonical neurons, which are thought to be involved in gating
mechanisms, selecting the feedback circuit best suited for the object to be
grasped. To be more specific, after LGM had mastered grasp learning, when we
investigated the neural responses of LGM layers we found units that are
selectively active for specific object sizes and orientations. Thus, we
concluded that these were the canonical neurons of LGM. In parallel,
Chapter 7 hypothesized that F5 canonical neurons implement a gating or priming
mechanism based on the object affordance. For example, given a small object the
LGM canonical neuron with small object preference would become active (as part
of its grasp planning task, as shown in Chapter 6) priming the visual feedback
circuit (a subpopulation of F5 purely motor and mirror neurons) that is best
suited for the control for small object grasping (e.g. the visual servo circuit
that is specialized for precision grip).
Although not modeled, a learning mechanism is proposed for
the gating mechanism as well. The canonical neurons not only prime the F5
visual feedback circuit for grasp execution but also they prime the feedback
circuits for learning ensuring that the neurons in a particular circuit will learn
to control specific grasps that are directed to objects for which the
canonical neuron has selectivity.
ILGM simulations of Chapter 5 showed that even without
object affordance, a variety of grips, including the precision grip, can be
learnt by actively interacting with the environment predicting the results of
the study of Butterworth et al. (1997).
We showed that task constraints are influential in shaping
the grasp repertoire of infants via the cube on the table task, which
together with other simulation results enabled us to make useful
predictions. To be precise, Chapter 5
1.
Predicted that infants in the period of early grasping, can
perform precision grasps
2.
Showed that task constraints shape motor development supporting
the view that development of precision grips is mediated by task constraints.
3.
Showed that object affordances could be represented in
infants’ grasp repertoire in spite the fact that the grasp planning circuit was
unable to access/extract object affordance information. This result is
important because it implies that tactile learning can train the visuomotor
learning in infancy.
4.
Predicted (as a corollary to 3) that object (visual)
affordances would be heavily represented in the motor and sensory areas of
cerebral cortex for objects that are manipulated often.
The simulation results of emergent object selectivity in LGM
and the analysis of what it takes to combine movement parameters into a
coherent whole for a successful grasp enabled us to produce nontrivial testable
predictions.
Specifically, we predicted that area F2 must be having
visually triggered neurons that are selective for object orientations.
Furthermore, we suggested that the area must have control over wrist
rotations. We further predicted that F5 canonical neurons would have tuning
fields according to the object orientation, similar to AIP visual dominant
neurons (Sakata et al. 1999). Therefore, we asserted that
a closer examination of area F2 and F5 canonical neurons would reveal orientation
selectivity either in isolation or in the form of gain fields over existing
object selectivity.
We generalized the above prediction and proposed a
systematic way of interpreting neural activities to amend the shortcomings of
analyses based on behaviour and neural firing correlation paradigm.
If some population of neurons were to specify a grasp plan
partially (by being responsible for one parameter of the plan), a learning mechanism
had to exist for shaping the connections between regions that specify the
complementary grasp parameters. This implies that in order to specify a grasp
plan F5 canonical neurons must have ‘gain fields’ based on other brain regions’
activity (the ‘gain fields’ would, behaviorally appear to be based on movement
parameters). Let us review the hypothetical experimenter we introduced in
Chapter 6 to present our proposed systematic approach.
If the experimenter finds out that area A is responsible for
some aspect (wrist orientation) of a complex movement (reach-to-grasp) and that
area B is responsible for controlling some other aspect (reach direction) of
the movement and if the activities of A and B are true control variables
determining the movement then we claim that
(1) The region A and B must be anatomically connected (the
connection may be a multi staged one, such as A connected to C, C connected to
B)
(2) The synaptic strength of the connection between A and B
encodes the movement generation (grasping) strategy of the grasping circuit
(3) If it is found that the connection between A and B is
unidirectional (say from A to B) then (from the viewpoint of the experimenters)
the activity of B neurons must be tuned by the population activity A.
(4) The verification of (3) can be used to decipher the
organization of visuomotor transformation circuit by recording simultaneously
from A and B. If activity of A predicts the activity of B better than the other
way round (i.e. activity of B predicts the activity of A) then the underlying
principle is that area A selects movement parameters, and B, based on the
selection of A, generates other movement parameter(s) completing (or further
specifying) the movement plan.
We hereby explicitly suggest a methodology to uncover the
roles of premotor areas in reach-to-grasp movements. For a true understanding
of premotor function, we strongly suggest that the premotor regions of F2, F5
bank (canonical neurons), F4 and wrist rotation encoding neurons of motor
cortex (F1) must be investigated with simultaneous recording
experiments. A feasible experimental design would be to set up sessions for
pair-wise recording of areas F2, F4, F5 and F1, in which the monkey would be
asked to reach and grasp for objects at various locations with changing
orientations. We claim that the rigorous analyses of neural firing, kinematics
of the hand and the object condition will reveal the mechanisms of grasp
learning and planning by showing the structural and functional dependency of
premotor regions in specifying grasp parameters.
The crucial feature of
LGM inherited from the architecture we developed in Chapter 4 was that it could
represent and produce a variety of grasp plans conditioned on input,
effectively learning a repertoire of grasp plans. The reinforcement framework
we adapted in Chapter 4 gives LGM the flexibility to include soft constraints
in grasp evaluation such that the grasps that are not favorable (due to
discomfort, excess energy consumption, etc.) are eliminated or represented with
low probability. Thus the reinforcement signal of Chapter 4, (joy of
grasping of Chapter 5 and neural grasp stability of Chapter 6) can
incorporate the anatomical and environmental constraints which are
important in shaping grasp development (Newell et al. 1989). The reinforcement signal can be viewed as the adaptive
value of action that shapes the movement repertoire through selection (Sporns and Edelman 1993).
The simulation results of Chapter 5 and 6 showed that the
architecture we introduced was adequate for grasp learning by producing
testable prediction for both infant development and for monkey neurophysiology.
We showed the neural network competency of LGM by demonstrating its
generalization ability over novel input conditions while retaining the property
of representing multiple actions for a given input.
While infants learn how to shape their reaching movements
into grasping actions, they also observe the visual stimuli they generate. The
main hypothesis of the MNS model was that the visual stimuli from the
successful grasps were used to train the mirror neurons. To this date, there is
no study pinpointing any stage in development when the mirror neurons start to
function fully. Our suggestion is that the MNS learning and grasp-learning
overlap. The amount of the overlap may have important consequences on the
organization of the two circuits. A very early learning in MNS may result in
mirror neurons that respond to premature grasping actions, as the training
stimuli will be based on the premature grasping attempts. A very late learning
may result in delayed visuomotor development in the infant. The effect of the
overlap amount must be studied via simulation studies to produce predictions to
be tested by the experimenters. These studies will stimulate research on the
development of mirror neurons, which is a lacking component of current mirror
neuron research.
The MNS model was based on grasping movements and excluded
some actions such as tearing and twisting for which mirror activity has been
observed. The MNS model must be extended with the help of new neurophysiological
studies to account for other hand manipulation actions. In essence, the hand
state we introduced must be extended to account for other actions. Although
the first step would be manually crafting the representation for the hand state
to cover the excluded hand manipulation actions, the major research direction
would be to model the mechanisms of how the brain learns to extract such a
representation from the visual world. We have already outlined one alternative
by claiming that the hand state is used as the visual feedback for hand
manipulation actions. Thus, a computational model showing how a visual feedback
mechanism may be bootstrapped by biologically realistic neural circuits, would
provide a general theory of mirror neuron development as well as explaining the
existence of mirror neuron system. Chapter 7 presented alternative feedback
mechanisms for grasping focusing on a single grasp type, namely the precision
grasp. The models there viewed with LGM (Chapters 5 and 6) should serve as a
starting point for the interested researcher. As shown in Chapters 5 and 6, the
infant/model learns how to make open-loop reach and grasp movements via goal
directed trial and error learning. Then the circuits involved in visual
feedback control are built based on the observation of the self-executed
ballistic grasps.
Infants’ early reaching and grasping movements are ballistic
and usually require correction after contact with the object. Infants use the
tactile feedback to conform their hands to the shape of the object reducing the
number of trials they need to perform for adapting their ballistic reach and
grasp planning. Learning to Grasp Models that we have presented in Chapters 5
and 6 did not include the tactile correction phase and required large number of
grasping actions for learning. The modeling of tactile feedback based grasping
is not trivial and requires (1) a detailed physical modeling of the hand and
the objects in terms of friction and contact forces and (2) a realistic model
of mechanoreceptor signals at the spinal and cortical levels. When combined
with learning to grasp models of Chapters 5 and 6, the tactile feedback
modeling research will provide a theory of how the tactile exploration can
train a visuomotor mapping circuit, and generate predictions on the grasp
development at the cortical and spinal level.
The neurophysiological findings assure that the visual
affordances of objects are represented in the monkey brain. We extended the
monkey studies to humans by assuming that object affordances were available to
the grasp programming centers and mirror neuron system without addressing the
issue of how and why the affordances were extracted from the vision of objects.
The how part is one of the major problems of computer vision, namely
feature extraction and object recognition. The why part is the question
of selecting the relevant features of an object for the motor task. The
natural extension of the learning studies presented in this thesis is to model
learning of what feature is relevant given a set of features. The
research on how to learn to associate relevant features of objects with the
movement parameters will present a more thorough view of the neural mechanisms
of the visuomotor transformation that takes place for hand manipulation
actions.
The arm/hand model we used was a kinematics chain, which was
articulated by specifying the angles of each joint. A more realistic arm/hand
would include muscles that are controlled with neural signals from the spinal
cord and the cortex. Such an arm/hand system would allow research on the
effects of corticospinal pathway immaturity during infancy.
Using the schema methodology, we focused on various brain
regions and implemented them with various levels of biological realism. A major
contribution would be to replace the schemas that have no neural implementation
with realistic neural circuits. For example, the visual analysis of the hand
was implemented using computer vision techniques with no neural implementation.
In addition, the affordances of the presented objects were encoded without
actually performing feature extraction. Thus, the two visual processes, the
visual analysis of the hand and the object, stand as future research
challenges.
While modeling the core mirror circuit of MNS model, we
noted that the core mirror circuit could be adapted using any supervised
learning technique. The range of the neurophysiological predictions would be
greatly extended by implementing the supervised learning in terms of realistic
neural circuits. In particular, the temporal to spatial conversion required for
the current implementation could be avoided.
Depending on future experiments that address the timings of
mirror neuron activities, a revision of the MNS model might be required. The
current modeling presented in this dissertation confronted experimentalists
with a range of nontrivial predictions that should stimulate experimenters in conducting
new experiments for testing the timing and population level activity of the
neurons involved in mirror neuron system and grasp planning, which in turn,
must be factored into the revisions of the models presented in this
dissertation.
In many of the presented simulation experiments, the
parameter selection was based on empirical tests. The dissertation studied a
broad range of functionality with paying less attention to the effect of
parameter selection on learning. It was empirically observed that the
parameters were not very sensitive in the sense that the models would not
collapse with small variations of the parameters. However, the result of
learning may show some differences according to the variations of the
parameters. For example when studying the learning to grasp, we did not analyze
the dependency of the acquired ‘grasp menu’ properties to the simulation
parameters. This study requires establishing a method to quantify the
properties of the ‘grasp menu’. Then using an extensive set of simulations, the
dependency can be formulated. For example, one property of a ‘grasp menu’ can
be chosen as the ratio between the number of power grasps and the precision
grasps acquired.
Akoev GN, Alekseev NP, Krylov BV (1988)
Mechanoreceptors, their functional organization. Springer-Verlag, Berlin ; New
York
Andersen RA, Asanuma C, Essick G, Siegel RM (1990)
Corticocortical connections of anatomically and physiologically defined
subdivisions within the inferior parietal lobule. Journal of Comparative
Neurology 296: 65-113
Andersen RA, Snyder LH, Bradley DC, Xing J (1997)
Multimodal representation of space in the posterior parietal cortex and its use
in planning movements. Annual Review of Neuroscience 20: 303-330
Anderson CH (1994) Basic elements of biological
computational systems. Int J Mod Phys C 5: 313-315
Arbib MA (1981) Perceptual structures and distributed motor
control. In: Brooks VB (ed) Handbook of physiology, section 2: The nervous
system, vol ii, motor control, part 1. American Physiological Society, pp
1449-1480
Arbib MA (1989) The metaphorical brain 2 : Neural networks
and beyond. Wiley, New York, N.Y.
Arbib MA (2001) The mirror system, imitation, and evolution
of language. In: Nehaniv C, Dautenhahn K (eds) Imitation in animals and
artifacts. The MIT Press
Arbib MA, âErdi P, Szentâagothai J (1998) Neural
organization : Structure, function, and dynamics. MIT Press, Cambridge, Mass.
Arbib MA, Hoff B (1994) Trends in neural modeling for reach
to grasp. In: Bennett KMB, Castiello U (eds) Insights into the reach to grasp
movement. North-Holland, Amsterdam ; New York, pp xxiii, 393
Armand J, Olivier E, Edgley SA, Lemon RN (1997) Postnatal
development of corticospinal projections from motor cortex to the cervical
enlargement in the macaque monkey. J Neurosci 17: 251-266
Atkeson CG (1989) Using local models to control movement.
In: Touretzky D (ed) Advances in neural information processing systems 1.
Morgan Kaufmann, San Mateo, CA, pp 157-183
Atkeson CG (1992) Memory-based approaches to approximating
continous functions. In: Casdagli M, Eubank S (eds) Nonlinear modeling and
forecasting. Addison Wesley, Redwood City, CA, pp 503-521
Atkeson CG, Schaal S (1995) Memory-based neural networks
for robot learning. Neurocomputing 9: 243-269
Barnes CL, Pandya DN (1992) Efferent cortical connections
of multimodal cortex of the superior temporal sulcus in the rhesus monkey.
Journal of Comparative Neurology 318: 222-244
Battaglia-Mayer A, Ferraina S, Mitsuda T, Marconi B,
Genovesio A, Onorati P, Lacquaniti F, Caminiti R (2000) Early coding of
reaching in the parietooccipital cortex. Journal of Neurophysiology 83:
2374-2391
Baud-Bovy G, Soechting JF (2001) Two virtual fingers in the
control of the tripod grasp. J Neurophysiol 86: 604-615
Bayley N (1936) The california infant scale of motor
development, birth to three years. University of California Press, Berkeley,
Calif.,
Bernstein NA (1967) The coordination and regulation of
movements. Pergamon Press, Oxford
Berthier NE, Clifton RK, Gullapalli V, McCall DD, Robin DJ
(1996) Visual information and object size in the control of reaching. J Motor
Behav 28: 187-197
Berthier NE, Clifton RK, McCall DD, Robin DJ (1999)
Proximodistal structure of early reaching in human infants. Exp Brain Res 127:
259-269
Bishop CM (1995) Neural networks for pattern recognition.
Clarendon Press ;Oxford University Press, Oxford New York
Bonda E, Petrides M, Ostry D, Evans A (1996) Specific
involvement of human parietal systems and the amygdala in the perception of
biological motion. J Neurosci 16: 3737-3744
Bortoff GA, Strick PL (1993) Corticospinal terminations in
2 new-world primates - further evidence that corticomotoneuronal connections
provide part of the neural substrate for manual dexterity. J Neurosci 13:
5105-5118
Bota M (2001) Neural homologies: Principles, databases and
modeling. In: Neurobiology. University of Southern California, Los Angeles
Boussaoud D, Ungerleider LG, Desimone R (1990) Pathways for
motion analysis - cortical connections of the medial superior temporal and
fundus of the superior temporal visual areas in the macaque. J Comp Neurol 296:
462-495
Bradley NS (2000) Motor control: Developmental aspects of
motor control in skill acquisition. In: Campbell SK, Vander Linden DW, Palisano
RJ (eds) Physical therapy for children. Saunders, Philadelphia, pp xvi, 1006
Bradley NS (2002) Postural control is crucial in
determining infant abilities. In, Los Angeles
Bremmer F, Graf W, Ben Hamed S, Duhamel JR (1999) Eye
position encoding in the macaque ventral intraparietal area (vip). NeuroReport
10: 873-878
Breteler MDK, Gielen SCAM, Meulenbroek RGJ (2001) End-point
constraints in aiming movements: Effects of approach angle and speed. Biol
Cybern 85: 65-75
Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L,
Gallese V, Seitz RJ, Zilles K, Rizzolatti G, Freund HJ (2001) Action
observation activates premotor and parietal areas in a somatotopic manner: An
fmri study. Eur J Neurosci 13: 400-404
Butterworth G, Verweij E, Hopkins B (1997) The development
of prehension in infants: Halverson revisited. Brit J Dev Psychol 15: 223-236
Caminiti R, Genovesio A, Marconi B, Mayer AB, Onorati P,
Ferraina S, Mitsuda T, Giannetti S, Squatrito S, Maioli MG, Molinari M (1999)
Early coding of reaching: Frontal and parietal association connections of
parieto-occipital cortex. European Journal of Neuroscience 11: 3339-3345
Caminiti R, Johnson PB, Burnod Y, Galli C, Ferraina S
(1990) Shift of preferred directions of premotor cortical cells with arm
movements performed across the workspace. Exp Brain Res 83: 228-232
Caminiti R, Johnson PB, Galli C, Ferraina S, Burnod Y
(1991) Making arm movements within different parts of space: The premotor and
motor cortical representation of a coordinate system for reaching to visual
targets. Journal of Neuroscience 11: 1182-1197
Cavada C, Goldmanrakic PS (1989) Posterior parietal cortex
in rhesus-monkey .2. Evidence for segregated corticocortical networks linking
sensory and limbic areas with the frontal-lobe. J Comp Neurol 287: 422-445
Cavada C, Goldman-Rakic PS (1989) Posterior parietal cortex
in rhesus monkey: I. Parcellation of areas based on distinctive limbic and
sensory corticocortical connections. J Comp Neurol 287: 393-421
Cisek P, Kalaska JF (2002) Simultaneous encoding of
multiple potential reach directions in dorsal premotor cortex. Journal of
Neurophysiology 87: 1149-1154
Clifton RK, Muir DW, Ashmead DH, Clarkson MG (1993) Is
visually guided reaching in early infancy a myth. Child Dev 64: 1099-1110
Clifton RK, Rochat P, Robin DJ, Berthier NE (1994)
Multimodal perception in the control of infant reaching. J Exp Psychol Human
20: 876-886
Colby CL, Duhamel JR (1991) Heterogeneity of extrastriate
visual areas and multiple parietal areas in the macaque monkey.
Neuropsychologia 29: 517-537
Colby CL, Duhamel JR (1996) Spatial representations for
action in parietal cortex. Cognitive Brain Research 5: 105-115
Colby CL, Duhamel JR, Goldberg ME (1993a) Ventral
intraparietal area of the macaque - anatomic location and visual response
properties. J Neurophysiol 69: 902-914
Colby CL, Duhamel JR, Goldberg ME (1993b) Ventral
intraparietal area of the macaque: Anatomic location and visual response
properties. J Neurophysiol 69: 902-914
Colby CL, Goldberg ME (1999) Space and attention in
parietal cortex. Annual Review of Neuroscience 22: 319-349
Constantinidis C, Steinmetz MA (1996) Neuronal activity in
posterior parietal area 7a during the delay periods of a spatial memory task.
Journal of Neurophysiology 76: 1352-1355
Constantinidis C, Steinmetz MA (2001) Neuronal responses in
area 7a to multiple-stimulus displays: I. Neurons encode the location of the
salient stimulus. Cerebral Cortex 11: 581-591
Corbetta D, Thelen E, Johnson K (2000) Motor constraints on
the development of perception-action matching in infant reaching. Infant Behav
Dev 23: 351-374
Crammond DJ, Kalaska JF (1996) Differential relation of
discharge in primary motor cortex and premotor cortex to movements versus
actively maintained postures during a reaching task. Exp Brain Res 108: 45-61
Debowy DJ, Ghosh S, Ro JY, Gardner EP (2001) Comparison of
neuronal firing rates in somatosensory and posterior parietal cortex during
prehension. Experimental Brain Research 137: 269-291
Decety J, Grezes J, Costes N, Perani D, Jeannerod M, Procyk
E, Grassi F, Fazio F (1997) Brain activity during observation of actions.
Influence of action content and subject's strategy. Brain 120: 1763-1777
Denny-Brown D (1950) Disintegration of motor function
resulting from cerebral lesions. Journal of Nervous and Mental Disease 112:
1-45
Desmurget M, Grea H, Prablanc C (1998) Final posture of the
upper limb depends on the initial position of the hand during prehension
movements. Exp Brain Res 119: 511-516
di Pellegrino G, Wise SP (1991) A neurophysiological
comparison of three distinct regions of the primate frontal lobe. Brain 114:
951-978
Dipellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti
G (1992) Understanding motor events - a neurophysiological study. Exp Brain Res
91: 176-180
Dong WK, Chudler EH, Sugiyama K, Roberts VJ, Hayashi T
(1994) Somatosensory, multisensory, and task-related neurons in cortical area
7b (pf) of unanesthetized monkeys. Journal of Neurophysiology 72: 542-564
Driscoll JA (2000) Comparison of neural network
architectures for the modeling of robot inverse kinematics. In: IEEE
Southeastcon, pp 44-51
D'Souza A, Vijayakumar S, Schaal S (2001) Learning inverse
kinematics. In: IEEE/RSJ International Conference on Intelligent Robots and
Systems, vol 1, pp 298 -303
Duhamel JR, Bremmer F, BenHamed S, Graf W (1997) Spatial
invariance of visual receptive fields in parietal cortex neurons. Nature 389:
845-848
Duhamel JR, Colby CL, Goldberg ME (1998) Ventral
intraparietal area of the macaque: Congruent visual and somatic response
properties. J Neurophysiol 79: 126-136
Dum RP, Strick PL (1991) The origin of corticospinal
projections from the premotor areas in the frontal lobe. J Neurosci 11: 667-689
Ehrsson HH, Fagergren A, Jonsson T, Westling G, Johansson
RS, Forssberg H (2000) Cortical activity in precision- versus power-grip tasks:
An fmri study. J Neurophysiol 83: 528-536
Eskandar EN, Assad JA (1999) Dissociation of visual, motor
and predictive signals in parietal cortex during visual guidance. Nature
Neuroscience 2: 88-93
Fadiga L, Fogassi L, Gallese V, Rizzolatti G (2000)
Visuomotor neurons: Ambiguity of the discharge or 'motor' perception? Int J
Psychophysiol 35: 165-177
Fadiga L, Fogassi L, Pavesi G, Rizzolatti G (1995) Motor
facilitation during action observation - a magnetic stimulation study. J
Neurophysiol 73: 2608-2611
Fagard J (2000) Linked proximal and distal changes in the
reaching behavior of 5- to 12- month-old human infants grasping objects of
different sizes. Infant Behav Dev 23: 317-329
Fagg AH, Arbib MA (1998) Modeling parietal-premotor
interactions in primate control of grasping. Neural Networks 11: 1277-1303
Fearing RS (1986) Simplified grasping and manipulation with
dexterous robot hands. IEEE Journal of Robotics and Automation 2: 188-195
Ferraina S, Battaglia-Mayer A, Genovesio A, Marconi B,
Onorati P, Caminiti R (2001) Early coding of visuomanual coordination during
reaching in parietal area pec. Journal of Neurophysiology 85: 462-467
Ferraina S, Garasto MR, Battaglia-Mayer A, Ferraresi P,
Johnson PB, Lacquaniti F, Caminiti R (1997a) Visual control of hand-reaching
movement: Activity in parietal area 7m. European Journal of Neuroscience 9:
1090-1095
Ferraina S, Johnson PB, Garasto MR, Battaglia-Mayer A,
Ercolani L, Bianchi L, Lacquaniti F, Caminiti R (1997b) Combination of hand and
gaze signals during reaching: Activity in parietal area 7 m of the monkey.
Journal of Neurophysiology 77: 1034-1038
Flament D, Hall EJ, Lemon RN (1992) The development of
cortico-motoneuronal projections investigated using magnetic brain-stimulation
in the infant macaque. J Physiol-London 447: 755-768
Flash T, Sejnowski TJ (2001) Computational approaches to
motor control. Curr Opin Neurobiol 11: 655-662
Fogassi L (1999) Mirror-like neurons in area 7b. In,
personal communication. Parma, Italy
Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L,
Rizzolatti G (2001) Cortical mechanism for the visual guidance of hand grasping
movements in the monkey - a reversible inactivation study. Brain 124: 571-586
Fogassi L, Gallese V, Dipellegrino G, Fadiga L, Gentilucci
M, Luppino G, Matelli M, Pedotti A, Rizzolatti G (1992) Space coding by
premotor cortex. Exp Brain Res 89: 686-690
Fogassi L, Gallese V, Fadiga L, Luppino G, Matelli M,
Rizzolatti G (1996) Coding of peripersonal space in inferior premotor cortex
(area f4). J Neurophysiol 76: 141-157
Fogassi L, Gallese V, Fadiga L, Rizzolatti G (1998) Neurons
responding to the sight of goal-directed hand/arm actions in the parietal area
pf (7b) of the macaque monkey. In: 28th Annual Meeting of Society for
Neuroscience, Los Angeles
Fogassi L, Raos V, Franchi G, Gallese V, Luppino G, Matelli
M (1999) Visual responses in the dorsal premotor area f2 of the macaque monkey.
Exp Brain Res 128: 194-199
Galea MP, Darian-Smith I (1995) Postnatal maturation of the
direct corticospinal projections in the macaque monkey. Cereb Cortex 5: 518-540
Gallese V (2002) Majority of hand related f5 neurons are
purely motor related. In, Los Angeles
Gallese V, Fadiga L, Fogassi L, Rizzolatti G (1996) Action
recognition in the premotor cortex. Brain 119: 593-609
Gallese V, Murata A, Kaseda M, Niki N, Sakata H (1994)
Deficit of hand preshaping after muscimol injection in monkey parietal cortex.
Neuroreport 5: 1525-1529
Gardner EP, Ro JY, Debowy D, Ghosh S (1999) Facilitation of
neuronal activity in somatosensory and posterior parietal cortex during
prehension. Exp Brain Res 127: 329-354
Gentilucci M, Fogassi L, Luppino G, Matelli M, Camarda R,
Rizzolatti G (1988) Functional organization of inferior area 6 in the macaque
monkey. I. Somatotopy and the control of proximal movements. Exp Brain Res 71:
475-490
Georgopoulos AP (1986) Neuronal population coding of
movement direction. Science 233: 1416-1419
Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT (1982)
On the relations between the direction of two-dimensional arm movements and
cell discharge in primate motor cortex. Journal of Neuroscience 2: 1527-1537
Geyer S, Matelli M, Luppino G, Zilles K (2000) Functional
neuroanatomy of the primate isocortical motor system. Anat Embryol 202: 443-474
Ghahramani Z, Wolpert DM, Jordan MI (1996) Generalization
to local remappings of the visuomotor coordinate transformation. J Neurosci 16:
7085-7096
Ghosh S, Brinkman C, Porter R (1987) A quantitative study
of the distribution of neurons projecting to the precentral motor cortex in the
monkey (m. Fascicularis). Journal of Comparative Neurology 259: 424-444
Gibson EJ (1969) Principles of perceptual learning and
development. Prentice-Hall, Englewood Cliffs, NJ
Gibson EJ (1988) Exploratory behavior in the development of
perceiving, acting and acquiring of knowledge. Annual Review of Psychology 39:
1-41
Gibson JJ (1966) The senses considered as perceptual
systems. Houghton Mifflin, Boston,
Gnadt JW, Andersen RA (1988) Memory related motor planning
activity in posterior parietal cortex of macaque. Exp Brain Res 70: 216-220
Gomi H, Kawato M (1993) Neural-network control for a
closed-loop system using feedback-error-learning. Neural Networks 6: 933-946
Gottlieb JP, Kusunoki M, Goldberg ME (1998) The
representation of visual salience in monkey parietal cortex. Nature 391:
481-484
Grafton ST, Arbib MA, Fadiga L, Rizzolatti G (1996)
Localization of grasp representations in humans by positron emission tomography
.2. Observation compared with imagination. Exp Brain Res 112: 103-111
Grafton ST, Fadiga L, Arbib MA, Rizzolatti G (1997)
Promotor cortex activation during observation and naming of familiar tools.
Neuroimage 6: 231-236
Grea H, Desmurget M, Prablanc C (2000) Postural invariance
in three-dimensional reaching and grasping movements. Exp Brain Res 134:
155-162
Halverson HM (1931) An experimental study of prehension in
infants by means of systematic cinema records. Genetic Psychology Monographs
10: 107-285
Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S,
Rizzolatti G (1998) Activation of human primary motor cortex during action
observation: A neuromagnetic study. Proceedings of the National Academy of
Sciences of the United States of America 95: 15061-15065
Haruno M, Wolpert DM, Kawato M (2001) Mosaic model for
sensorimotor learning and control. Neural Comput 13: 2201-2220
Hertz J, Palmer RG, Krogh A (1991) Introduction to the
theory of neural computation. Addison-Wesley Pub. Co., Redwood City, Calif.
Hinde RA, Rowell TE (1964) Behavior socially living rhesus
monkeys in their first six months. Proc Zool Soc Lond 143: 609-649
Hoff B, Arbib MA (1993) Models of trajectory formation and
temporal interaction of reach and grasp. J Motor Behav 25: 175-192
Holden EJ (1997) Visual recognition of hand motion. In:
Computer Science. University of Western Australia
Hoshi E, Tanji J (2000) Integration of target and body-part
information in the premotor cortex when planning action. Nature 408: 466-470
Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC,
Rizzolatti G (1999) Cortical mechanisms of human imitation. Science 286:
2526-2528
Iberall T, Arbib MA (1990) Schemas for the control of hand
movements: An essay on cortical localization. In: M.A. G (ed) Vision and
action: The control of grasping. Ablex, Norwood, NJ
Jeannerod M, Arbib MA, Rizzolatti G, Sakata H (1995)
Grasping objects - the cortical mechanisms of visuomotor transformation. Trends
Neurosci 18: 314-320
Jeannerod M, Decety J (1990) The accuracy of visuomotor
transformation: An investigation into the mechanisms of visual recognition of
objects. In: Goodale MA (ed) Vision and action : The control of grasping. Ablex
Pub. Corp., Norwood, N.J., pp viii, 367
Jeannerod M, Paulignan Y, Weiss P (1998) Grasping an
object: One movement, several components. Novartis Foundation Symposium 218:
5-16; discussion 16-20
Johansson RS, Westling G (1987a) Signals in tactile
afferents from the fingers eliciting adaptive motor responses during precision
grip. Experimental Brain Research 66: 141-154
Johansson RS, Westling G (1987b) Significance of cutaneous
input for precise hand movements. Electroencephalography & Clinical
Neurophysiology - Supplement 39: 53-57
Johansson RS, Westling G, Backstrom A, Flanagan JR (2001)
Eye-hand coordination in object manipulation. Journal of Neuroscience 21:
6917-6932
Jordan MI, Rumelhart DE (1992) Forward models - supervised
learning with a distal teacher. Cognitive Sci 16: 307-354
Kakei S, Hoffman DS, Strick PL (1999) Muscle and movement
representations in the primary motor cortex. Science 285: 2136-2139
Kakei S, Hoffman DS, Strick PL (2001) Direction of action
is represented in the ventral premotor cortex. [see comments.]. Nat Neurosci 4:
1020-1025
Kandel ER, Schwartz JH, Jessell TM (2000) Principles of
neural science. McGraw-Hill Health Professions Division, New York
Karniel A, Inbar GF (1997) A model for learning human
reaching movements. Biol Cybern 77: 173-183
Kawato M, Furukawa K, Suzuki R (1987) A hierarchical neural
neural network model for control and learning of voluntary movement. Biological
Cybernetics 57: 169-185
Kawato M, Gomi H (1992) A computational model of 4 regions
of the cerebellum based on feedback-error learning. Biological Cybernetics 68:
95-103
Kincaid D, Cheney EW (1991) Numerical analysis :
Mathematics of scientific computing. Brooks/Cole, Pacific Grove, Calif.
Klein CA, Chujenq C, Ahmed S (1995) A new formulation of
the extended jacobian method and its use in mapping algorithmic singularities
for kinematically redundant manipulators. Ieee T Robotic Autom 11: 50-55
Krams M, Rushworth MF, Deiber MP, Frackowiak RS, Passingham
RE (1998) The preparation, execution and suppression of copied movements in the
human brain. Exp Brain Res 120: 386-398
Lambert P, Carron T (1999) Symbolic fusion of
luminance-hue-chroma features for region segmentation. Pattern Recogn 32:
1857-1872
Lantz C, Melen K, Forssberg H (1996) Early infant grasping
involves radial fingers. Dev Med Child Neurol 38: 668-674
Lassek A (1954) The pyramidal tract: Its status in
medicine. Charles C. Thomas, Springfield, Illinois
Lawrence DG, Hopkins DA (1976) The development of motor
control in the rhesus monkey: Evidence concerning the role of
corticomotoneuronal connections. Brain 99: 235-254
Lewis JW, Van Essen DC (2000) Corticocortical connections
of visual, sensorimotor, and multimodal processing areas in the parietal lobe
of the macaque monkey. Journal of Comparative Neurology 428: 112-137
Leydold J, Hormann W (2000) Universal algorithms as an
alternative for generating non-uniform continuous random variates. In: Proc
International Conference on Monte Carlo Simulation
Lockman J, Ashmead DH, Bushnell EW (1984) The development
of anticipatory hand orientation during infancy. Journal of Experimental Child
Psychology 37: 176-186
Lowe DG (1991) Fitting parameterized 3-dimensional models
to images. Ieee T Pattern Anal 13: 441-450
Luppino G, Matelli M, Camarda RM, Gallese V, Rizzolatti G
(1991) Multiple representations of body movements in mesial area-6 and the
adjacent cingulate cortex - an intracortical microstimulation study in the
macaque monkey. J Comp Neurol 311: 463-482
Luppino G, Murata A, Govoni P, Matelli M (1999) Largely
segregated parietofrontal connections linking rostral intraparietal cortex
(areas aip and vip) and the ventral premotor cortex (areas f5 and f4). Exp
Brain Res 128: 181-187
MacKay WA (1992) Properties of reach-related neuronal
activity in cortical area 7a. Journal of Neurophysiology 67: 1335-1345
MacKenzie CL, Iberall T (1994) The grasping hand.
North-Holland, Amsterdam ; New York
Maioli MG, Squatrito S, Samolsky-Dekel BG, Sanseverino ER
(1998) Corticocortical connections between frontal periarcuate regions and
visual areas of the superior temporal sulcus and the adjoining inferior
parietal lobule in the macaque monkey. Brain Res 789: 118-125
Marconi B, Genovesio A, Battaglia-Mayer A, Ferraina S,
Squatrito S, Molinari M, Lacquaniti F, Caminiti R (2001) Eye-hand coordination
during reaching. I. Anatomical relationships between parietal and frontal
cortex. Cerebral Cortex 11: 513-527
Marteniuk RG, MacKenzie CL (1990) Invariance and
variability in human prehension: Implications for theory development. In:
Goodale MA (ed) Vision and action: The control of grasping 163-180, Norwood, NJ: Ablex
Matelli M (1984) Interconnections within the postarcuate cortex
(area 6) of the macaque monkey. Brain Res 310: 388-392
Matelli M (1986) Afferent and efferent projections of the
inferior area 6 in the macaque monkey. J Comp Neurol 251: 281-298
Matelli M, Luppino G, Fogassi L, Rizzolatti G (1989)
Thalamic input to inferior area-6 and area-4 in the macaque monkey. J Comp
Neurol 280: 468-488
Matelli M, Luppino G, Murata A, Sakata H (1994) Independent
anatomical circuits for reaching and grasping linking the inferior parietal
sulcus and inferior area 6 in macaque monkey. In: Society for Neuroscience, vol
20, p 404.404
Matelli M, Luppino G, Rizzolatti G (1991) Architecture of
superior and mesial area 6 and the adjacent cingulate cortex in the macaque
monkey. J Comp Neurol 311: 445-462
Maunsell JH (1995) The brain's visual world: Representation
of visual targets in cerebral cortex. Science 270: 764-769
Miall RC, Wolpert DM (1996) Forward models for
physiological motor control. Neural Networks 9: 1265-1279
Motter BC, Mountcastle VB (1981) The functional properties
of the light-sensitive neurons of the posterior parietal cortex studied in
waking monkeys: Foveal sparing and opponent vector organization. Journal of
Neuroscience 1: 3-26
Motter BC, Steinmetz MA, Duffy CJ, Mountcastle VB (1987)
Functional properties of parietal visual neurons: Mechanisms of directionality
along a single axis. Journal of Neuroscience 7: 154-176
Muir RB, Lemon RN (1983) Corticospinal neurons with a
special role in precision grip. Brain Research 261: 312-316
Murata A (2000) Parietal neurons discriminating allocentric
fixation locations. In, Kyoto, Japan
Murata A, Fadiga L, Fogassi L, Gallese V, Raos V,
Rizzolatti G (1997a) Object representation in the ventral premotor cortex (area
f5) of the monkey. J Neurophysiol 78: 2226-2230
Murata A, Fadiga L, Fogassi L, Gallese V, Rizzolatti G
(1997b) Visuomotor properties of grasping neurons of inferior area 6. Pflug
Arch Eur J Phy 434: 73-73
Murata A, Gallese V, Kaseda M, Sakata H (1996) Parietal
neurons related to memory-guided hand manipulation. J Neurophysiol 75:
2180-2186
Murata A, Gallese V, Luppino G, Kaseda M, Sakata H (2000)
Selectivity for the shape, size, and orientation of objects for grasping in
neurons of monkey parietal area aip. J Neurophysiol 83: 2580-2601
Neal JW, Pearson RC, Powell TP (1990) The ipsilateral
cortico-cortical connections of area 7b, pf, in the parietal and temporal lobes
of the monkey. Brain Research 524: 119-132
Newell KM (1986) Motor development in children: Aspects of
coordination and control. In: Wade MG, Whiting HTA (eds) Motor development in
children : Aspects of coordination and control. Nijhoff, Boston, pp 341-360
Newell KM, Scully DM, McDonald PV, Baillargeon R (1989)
Task constraints and infant grip configurations. Developmental Psychobiology
22: 817-831
Newsome WT, Britten KH, Movshon JA (1989) Neuronal
correlates of a perceptual decision. Nature 341: 52-54
Nishitani N, Hari R (2000) Temporal dynamics of cortical
representation for action. Proceedings of the National Academy of Sciences of
the United States of America 97: 913-918
O'Keefe J, Dostrovsky J (1971) The hippocampus as a spatial
map. Preliminary evidence from unit activity in the freely-moving rat. Brain
Research 34: 171-175
Olivier E, Edgley SA, Armand J, Lemon RN (1997) An
electrophysiological study of the postnatal development of the corticospinal
system in the macaque monkey. J Neurosci 17: 267-276
Oyama E, Agah A, Chong NY, Maeda T (2001a) Inverse
kinematics learning by modular architecture neural networks with performance
prediction networks. In: IEEE International
Conference on Robotics and Automation, vol 1
Oyama E, Agah A, MacDorman KF, Maeda T, Tachi S (2001b) A
modular neural network architecture for inverse kinematics model learning.
Neurocomputing 38: 797-805
Oztop E (2000) Neurobench. In, Los Angeles
Pandya DN, Seltzer B (1982) Intrinsic connections and
architectonics of posterior parietal cortex in the rhesus monkey. Journal of
Comparative Neurology 204: 196-210
Perret DI, Harries MH, Benson PJ, Chitty AJ, Mistlin AJ
(1990a) Retrieval of structure from rigid and biological motion: An analysis of
the visual responses of neurons in the macaque temporal cortex. In: Blake A,
Troscianko T (eds) Ai and the eye. John Wiley and Sons Ltd., pp 181-199
Perret DI, Mistlin AJ, Harries MH, Chitty AJ (1990b)
Understanding the visual appearance and consequence of hand actions. In:
Goodale MA (ed) Vision and action: The control of grasping 163-180, Norwood, NJ: Ablex, pp 163-180
Raos V, Franchi G, Fogassi L, Gallese V, Luppino G, Matelli
M (1998) Functional organization of area f2 in the monkey. Eur J Neurosci 10:
87-87
Ratcliff G (1991) Brain and space: Some deductions from
clinical evidence. In: Paillard J (ed) Brain and space. Oxfort University
Press, Oxfort England; New York
Rizzolatti G, Arbib MA (1998) Language within our grasp.
Trends Neurosci 21: 188-194
Rizzolatti G, Arbib MA (1999) From grasping to speech:
Imitation might provide a missing link - reply. Trends Neurosci 22: 152-152
Rizzolatti G, Camarda R, Fogassi L, Gentilucci M, Luppino
G, Matelli M (1988) Functional organization of inferior area 6 in the macaque
monkey. Ii. Area f5 and the control of distal movements. Exp Brain Res 71:
491-507
Rizzolatti G, Fadiga L (1998) Grasping objects and grasping
action meanings: The dual role of monkey rostraventral premotor cortex (area
f5). In: Sensory guidance of movement, novartis foundation symposium 218.
Wiley, Chichester, pp 81-103
Rizzolatti G, Fadiga L, Gallese V, Fogassi L (1996a)
Premotor cortex and the recognition of motor actions. Cognitive Brain Res 3:
131-141
Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E,
Perani D, Fazio F (1996b) Localization of grasp representations in humans by
pet .1. Observation versus execution. Exp Brain Res 111: 246-252
Rizzolatti G, Fogassi L, Gallese V (2000) Mirror neurons:
Intentionality detectors? Int J Psychol 35: 205-205
Rizzolatti G, Fogassi L, Gallese V (2001a)
Neurophysiological mechanisms underlying the understanding and imitation of
action. Nat Rev Neurosci 2: 661-670
Rizzolatti G, Gallese V (2001) Mirror neuron
electrophysiological recording data (raw). In. Personal Communication
Rizzolatti G, Gallese V, Fogassi L, Keysers C (2001b)
Experimental setup of mirror neuron recordings. In. Personal Communication
Rizzolatti G, Luppino G, Matelli M (1998) The organization
of the cortical motor system: New concepts. Electroen Clin Neuro 106: 283-296
Ro JY, Debowy D, Ghosh S, Gardner EP (2000) Depression of
neuronal firing rates in somatosensory and posterior parietal cortex during
object acquisition in a prehension task. Experimental Brain Research 135: 1-11
Robinson CJ, Burton H (1980a) Organization of somatosensory
receptive fields in cortical areas 7b, retroinsula, postauditory and granular
insula of m. Fascicularis. Journal of Comparative Neurology 192: 69-92
Robinson CJ, Burton H (1980b) Somatic submodality
distribution within the second somatosensory (sii), 7b, retroinsular,
postauditory, and granular insular cortical areas of m. Fascicularis. Journal
of Comparative Neurology 192: 93-108
Rochat P, Morgan R (1995) Spatial determinants in the
perception of self-produced leg movements by 3-5 month-old infants.
Developmental Psychology 31: 626-636
Rosenbaum DA (1991) Human motor control. Academic Press,
San Diego
Rosenbaum DA, Loukopoulos LD, Meulenbroek RG, Vaughan J, Engelbrecht
SE (1995) Planning reaches by evaluating stored postures. Psychological Review
102: 28-67
Rosenbaum DA, Meulenbroek RGJ, Vaughan J, Jansen C (1999)
Coordination of reaching and grasping by capitalizing on obstacle avoidance and
other constraints. Exp Brain Res 128: 92-100
Rosenbaum DA, Meulenbroek RJ, Vaughan J, Jansen C (2001)
Posture-based motion planning: Applications to grasping. Psychol Rev 108:
709-734
Rothwell JC (1994) Control of human voluntary movement.
Chapman & Hall, London ; New York
Roy AC, Paulignan Y, Farne A, Jouffrais C, Boussaoud D
(2000) Hand kinematics during reaching and grasping in the macaque monkey.
Behav Brain Res 117: 75-82
Rumelhart DE, Hinton GE, Williams RJ (1986) Learning
internal representations by error propagation. In: Rumelhart DE, McClelland JL,
group aP (eds) Parallel distributed processing, vol 1: Foundations, pp 151-193
Rushworth MF, Nixon PD, Passingham RE (1997) Parietal
cortex and movement. I. Movement selection and reaching. Experimental Brain
Research 117: 292-310
Russ JC (1998) The image processing handbook. CRC Press,
Boca Raton, FL
Sakata H, Taira M, Kusunoki M, Murata A, Tanaka Y (1997a)
The tins lecture - the parietal association cortex in depth perception and
visual control of hand action. Trends Neurosci 20: 350-357
Sakata H, Taira M, Kusunoki M, Murata A, Tanaka Y, Tsutsui
K (1998) Neural coding of 3d features of objects for hand action in the
parietal cortex of the monkey. Philos T Roy Soc B 353: 1363-1373
Sakata H, Taira M, Kusunoki M, Murata A, Tsutsui K, Tanaka
Y, Shein WN, Miyashita Y (1999) Neural representation of three-dimensional
features of manipulation objects with stereopsis. Exp Brain Res 128: 160-169
Sakata H, Taira M, Murata A, Gallese V, Tanaka Y, Shikata
E, Kusunoki M (1997b) Parietal visual neurons coding three-dimensional
characteristics of objects and their relation to hand action. In: Their P,
Karnath HO (eds) Parietal lobe contributions to orientation in 3d space.
Springer-Verlag, Heidelberg
Sakata H, Taira M, Murata A, Mine S (1995) Neural
mechanisms of visual guidance of hand action in the parietal cortex of the
monkey. Cereb Cortex 5: 429-438
Salimi I, Brochier T, Smith AM (1999a) Neuronal activity in
somatosensory cortex of monkeys using a precision grip. I. Receptive fields and
discharge patterns. Journal of Neurophysiology 81: 825-834
Salimi I, Brochier T, Smith AM (1999b) Neuronal activity in
somatosensory cortex of monkeys using a precision grip. Iii. Responses to
altered friction perturbations. Journal of Neurophysiology 81: 845-857
Sanger TD (1996) Probability density estimation for the
interpretation of neural population codes. J Neurophysiol 76: 2790-2793
Schaal S, Atkeson CG, Vijayakumar S (2002) Scalable
techniques from nonparametric statistics for real time robot learning. Applied
Intelligence (in press)
Sciavicco L, Siciliano B (2000) Modelling and control of
robot manipulators. Springer, London ; New York
Scott SH, Sergio LE, Kalaska JF (1997) Reaching movements
with similar hand paths but different arm orientations. Ii. Activity of
individual cells in dorsal premotor cortex and parietal area 5. J Neurophysiol
78: 2413-2426
Sergio LE, Kalaska JF (1997) Systematic changes in
directional tuning of motor cortex cell activity with hand location in the
workspace during generation of static isometric forces in constant spatial
directions. J Neurophysiol 78: 1170-1174
Shadlen MN, Newsome WT (1996) Motion perception: Seeing and
deciding. Proceedings of the National Academy of Sciences of the United States
of America 93: 628-633
Shadlen MN, Newsome WT (2001) Neural basis of a perceptual
decision in the parietal cortex (area lip) of the rhesus monkey. J Neurophysiol
86: 1916-1936
Shen LM, Alexander GE (1997a) Neural correlates of a
spatial sensory-to-motor transformation in primary motor cortex. J Neurophysiol
77: 1171-1194
Shen LM, Alexander GE (1997b) Preferential representation
of instructed target location versus limb trajectory in dorsal premotor area. J
Neurophysiol 77: 1195-1212
Siegel RM (1998) Representation of visual space in area 7a
neurons using the center of mass equation. Journal of Computational
Neuroscience 5: 365-381
Siegel RM, Read HL (1997) Analysis of optic flow in the
monkey parietal area 7a. Cerebral Cortex 7: 327-346
Smeets JB, Brenner E (1999) A new view on grasping. [see
comments.]. Motor Control 3: 237-271
Smeets JB, Brenner E (2001) Independent movements of the
digits in grasping. Experimental Brain Research 139: 92-100
Snippe HP (1996) Parameter extraction from population
codes: A critical assessment. Neural Comput 8: 511-529
Snyder LH, Batista AP, Andersen RA (1997) Coding of
intention in the posterior parietal cortex. [see comments.]. Nature 386:
167-170
Snyder LH, Batista AP, Andersen RA (2000) Intention-related
activity in the posterior parietal cortex: A review. Vision Research 40:
1433-1441
Sonka M, Hlavac V, Boyle R (1993) Image processing,
analysis, and machine vision. Chapman & Hall Computing, London ; New York
Sporns O, Edelman GM (1993) Solving bernstein's problem: A
proposal for the development of coordinated movement by selection. Child
Development 64: 960-981
Sporns O, Edelman GM, Meijer OG (1998) Bernstein's dynamic
view of the brain: The current problems of modern neurophysiology (1945). Motor
Control 2: 283-305
Sporns O, Tononi G, Edelman GM (2000) Connectivity and
complexity: The relationship between neuroanatomy and brain dynamics. Neural
Networks 13: 909-922
Stein JF (1991) Space and the parietal association areas.
In: Paillard J (ed) Brain and space. Oxfort University Press, Oxfort England;
New York
Streri A (1993) Seeing, reaching, touching : The relations
between vision and touch in infancy. Harvester Wheatsheaf, London ; New York
Sutton RS, Barto AG (1998) Reinforcement learning : An
introduction. MIT Press, Cambridge, Mass.
Taira M, Mine S, Georgopoulos AP, Murata A, Sakata H (1990)
Parietal cortex neurons of the monkey related to the visual guidance of hand
movement. Exp Brain Res 83: 29-36
Tang WS, Wang J (2001) A recurrent neural network for
minimum infinity-norm kinematic control of redundant manipulators with an
improved problem formulation and reduced architecture complexity. Ieee T Syst
Man Cy B 31: 98-105
Thelen E (2000) Motor development as foundation and future
of developmental psychology. Int J Behav Dev 24: 385-397
Triggs WJ, Yathiraj S, Young MS, Rossi F (1998) Effects of
task and task persistence on magnetic motor-evoked potentials. Journal of
Contemporary Neurology 1998 (electronic journal: http://scholar.lib.vt.edu/ejournals/JCN/ncn-mirror/articles/003/Triggs.pdf)
Twitchell TE (1970) Reflex mechanisms and the development
of prehension. In: Connolly KJ (ed) Mechanisms of motor skill development:.
Academic Press, London, New York
Umilta MA, Kohler E, Gallese V, Fogassi L, Fadiga L,
Keysers C, Rizzolatti G (2001) I know what you are doing: A neurophysiological
study. Neuron 31: 155-165
van der Meer AL, van der Weel FR, Lee DN (1995) The
functional significance of arm movements in neonates. Science 267: 693-695
von Hofsten C (1982) Eye-hand coordination in the newborn.
Developmental Psychology 18: 450-461
von Hofsten C (1984) Developmental changes in the
organization of prereaching movements. Developmental Psychology 20: 378-388
von Hofsten C (1993) The structuring of neonatal arm
movements. Child Dev 64: 1046-1057
von Hofsten C, Ronnqvist L (1988) Preparation for grasping
an object: A developmental study. Journal of Experimental Psychology: Human
Perception & Performance 14: 610-621
Whitney DE (1969) Resolved motion rate control of
maniplators and human prostheses. IEEE Transactions on Man-Machine Systems 10:
47-53
Williams RJ (1992) Simple statistical gradient-following
algorithms for connectionist reinforcement learning. Machine Learning 8:
229-256
Willis WD, Coggeshall RE (1991) Sensory mechanisms of the
spinal cord. Plenum Press, New York
Wise SP, Boussaoud D, Johnson PB, Caminiti R (1997)
Premotor and parietal cortex: Corticocortical connectivity and combinatorial
computations. Annual Review of Neuroscience 20: 25-42
Wolpert DM, Ghahramani Z (2000) Computational principles of
movement neuroscience. Nat Neurosci 3 Suppl: 1212-1217
Wolpert DM, Ghahramani Z, Flanagan JR (2001) Perspectives
and problems in motor learning. Trends Cogn Sci 5: 487-494
Wolpert DM, Kawato M (1998) Multiple paired forward and
inverse models for motor control. Neural Networks 11: 1317-1329
Wolpert DM, Miall RC, Kawato M (1998) Internal models in
the cerebellum. Trends Cogn Sci 2: 338-347
Wu CW, Bichot NP, Kaas JH (2000) Converging evidence from
microstimulation, architecture, and connections for multiple motor areas in the
frontal and cingulate cortex of prosimian primates. J Comp Neurol 423: 140-177
Zemel RS, Dayan P, Pouget A (1998) Probabilistic
interpretation of population codes. Neural Comput 10: 403-430
The system was implemented using Java programming language
on a Linux operating system. The grasp simulator can be accessed using the URL
http://www-clmc.usc.edu/erhan/models/MNS. The simulation applet at this URL
also includes a simplified version of the MNS model. The applet enables the
users to test the action recognition ability of the model.
The segmentation system as a whole works as follows:
1.
Start with N rectangles (called nodes), set thresholds for
red, green and blue variances as rV, gV, bV
2.
For each node calculate the red, green, blue variance as rv,
gv, bv
3.
If any of the variance is higher than the threshold (rv>rV
or gv>gV or bv>bV) then split the node into four equal pieces and apply
step 2 and step 3 recursively
4.
Feed in the mean red, green and blue values in that region to
the Color Expert to determine the color of the node.
5.
Make a list of nodes that are of the same color (add node to
the list reserved for that color).
6.
Repeat 2-5 until no split occurs.
7.
Cluster (in terms of Euclidean distance on the image) the
nodes and discard the outliers from the list (use the center of the node as the
position of the node). The discarding is performed either when a region is very
far from the current mean (weighted center) or it is not "connected"
to the current center position. The connectedness is defined as follows. The
regions A and B are connected if the points lying on the line segment joining
the centers of A and B are the same color as A and B. Once again, the Color
Expert is used to determine the percentage of the correct (colors of A and B)
colors lying on the line segment. If this percentage is over a certain
threshold (e.g. 70%) then the regions A and B are taken as
"connected". (This strategy would not work for a
"sausage-shaped" region, but does work for patches created by the
coloring we used in the glove.)
8.
For each pruned list (corresponding to a color) find the
weighted (by the area of the node) mean of the clusters (in terms of image
coordinate).
9.
Return the cluster mean coordinates as the segmented regions
center.
So we do not exactly perform the merge part of the
split-merge algorithm. The return values from this procedure are the (x,y) coordinates
of the center of color patches found. Another issue is how to choose the
thresholds. The variance values are not very critical. A too small value
increases computation time but does not affect the number of colors extracted
correctly (though the returned coordinates may be shifted slightly). To see why
intuitively, one can notice that the center of a rectangle and the centroid of
the centers of the quarter rectangles (say after a split operation) would be
the same. This means that if a region is split unnecessarily (because the
threshold variances were set to very small values) it is likely to be averaged
out with our algorithm since it is likely that the four split rectangles will
have the same color and will be connected (with our definition of connectedness)
·
Determine the opposition axis to grasp the object.
·
Compute the two (outer) points A and B at which the
opposition axis intersects the object surface. They serve as the contact points
for the virtual fingers that will be involved in the grasp.
·
Assign the real fingers to virtual fingers. The
particular heuristic we used in the experiments was the following. If the
object is on the right [left] with respect to the arm then the thumb is
assigned to the point A if A is on the left of [at a lower level than] B
otherwise the thumb is assigned to B. The index finger is assigned to the
remaining point.
·
Determine an approximate target position C, for the
wrist. Mark the target for the wrist on the line segment connecting the current
position of the wrist and the target for the thumb a fixed length (determined
by the thumb length) away from the thumb target.
·
Solve the inverse kinematics for only the wrist reach
(ignore the hand).
·
Solve the inverse kinematics for grasping. Using the
sum of distance squares of the finger tips to the target contact points do a
random hill climbing search to minimize the error. Note that the search starts
with placing the wrist at point C. However, the wrist position is not included
in the error term.
·
The search stops when the simulator finds a
configuration with error close to zero (success) or after a fixed number of
steps (failure to reach). In the success case the final configuration is
returned as the solution for the inverse kinematics for the grasp. Otherwise
failure-to-reach is returned.
Execute the reach and grasp. At this point the simulator
knows the desired target configuration in terms of joint angles. So what
remains to be done is to perform the grasp in a realistic way (in terms of
kinematics). The simplest way to perform the reach is to linearly change the
joint angles from the initial configuration to the target configuration. But
this does not produce a bell shaped velocity profile (nor exactly a constant
speed profile either because of the non-linearity in going from joint angles to
end effector position). The perfect way to plan an end-effector trajectory
requires the computation of the Jacobian. However we are not interested in
perfect trajectories as long as the target is reached with a bell-shaped
velocity profile. To get the effect it is usually sufficient to modify the idea
of linearly changing the joint angles little bit. We simply modulate the change
of time by replacing time with a third order polynomial that will match our
constraints for time (starts at 0 climbs up to 1 monotonically). Note that we
are still working in the joint space and our method may suffer from the
non-linearity in transforming the joint angles to end effector coordinates.
However, our empirical studies showed that a satisfactory result, for our
purposes, could be achieved in this way.
The simulation environment is developed using Java
programming language. The simulation environment consists of a 3D kinematics
arm model (see Chapter 3) and routines implementing the LGM circuit. Note that
from an implementation point of view the learning to grasp circuits of Chapters
5 and 6 are essentially the same. In this section, we present a brief overview
of the simulation environment. The further details are available at the URL
http://www-clmc.usc.edu/erhan/models/LGM.
The main behavior of the simulation system is determined by
the resource file ‘KolParameters.res’. Many simulation parameters can be set
within this file. However, some of the parameters are hard coded in the Java
class files comprising the simulation system. The 3D positions and vectors are
defined using a spherical reference frame. The PAR, MER and RAD tags are used
to indicate elevation, azimuth and radius components respectively.
Object position range parameters
define the range of the object position in the workspace. In the following
segment of text, the first two correspond to the range of the elevation; the
following two corresponds to the range of azimuth and finally the last two
corresponds to the range of radius.
minPAR -45
maxPAR 45
minMER -45
maxMER 45
minRAD 700
maxRAD 1300
Object location coding length
parameters define how many units to allocate for representing each position
component in the affordance layer. In the below setting, the object location
will be represented with 100 units.
obj_locPAR_code_len
10
obj_locMER_code_len
10
obj_locRAD_code_len
1
Object location coding variance
defines the variance for population encoding of the object location.
obj_encode_var
1
Object axis orientation range
parameters define the minimum and maximum allowed tilt of the object around
the z-axis (in the frontal plane). The number of units allocated for encoding
the tilts (in three coordinate axes) is hardwired in the file Motor.java
minTILT 0
maxTILT 90
Base learning rate parameter
is used as the common multiplier for all the learning rates in the grasp
learning circuit.
eta
0.5
LGM layer length parameters
define the number of units to allocate for the layers generating the motor
parameters. BANK, PITCH and HEADING tags indicate the supination-pronation,
wrist extension-flexion and radial/ulnar deviation movements respectively. (The
Virtual Fingers layer in these simulations is not engaged fully as it is
represented as a layer of ten units encoding the synergistic enclosure speed of
the hand.)
hand_rotBANK_code_len
9
hand_rotPITCH_code_len
9
hand_rotHEADING_code_len 1
The tags locMER, locPAR and locRAD
indicate the approach direction vector components.
hand_locMER_code_len
7
hand_locPAR_code_len
7
hand_locRAD_code_len
1
Learning session parameters
define the behavior of the simulator during learning. For a learning session,
the simulator makes MAXBABBLE number of reach/grasp attempts. For each
approach-direction, the simulator makes MAXROTATE grasping attempts. After
MAXREACH reaches are done, the next input condition is selected (e.g. the
object is placed in a different position). After each weightSave reach/grasp
trials the learned connections are written to disk. Note that MAXBABBLE only limits the maximum number of attempts
the simulator will make. A particular simulation may be stopped at any instant.
The saved connection weights then can be used for testing the performance at a
later time. Reach2Target parameter indicates which part of the hand should be
used in reaching for the object. The possible values are {INDEX, MIDDLE, THUMB}
x {0,1,2} where 2 indicates the tip.
MAXREACH 5
MAXROTATE 7
MAXBABBLE
10000
weightSave
4500
Reach2Target
INDEX1
Grasp stability parameters
define the acceptable grasps in terms of physical stability. costThreshold
specifies the allowable inaccuracy in grasping. Ideally, the cost of grasping
(a measure of the instability, see Chapter 5.5) should be small indicating that the grasp is
successful. Empirically, a value between 0.5 and 0.8 gives a good result for
the implemented cost function. If the distance of the touched object to the
palm is less than palmThreshold and the movement of the object due to finger
contact is towards the palm then the palm is used as a virtual finger to
counteract the force exerted by the fingers. The negReinforcement parameter
specifies the level of punishment returned when a grasp attempt fails.
Empirically values greater than –0.1 and less than 0 result in good learning.
Generally, a large negative reinforcement overwhelms the positively reinforced
plans before they have chance to get represented in the layers.
costThreshold
0.8
palmThreshold
150
negReinforcement
-0.05
Exploration and exploitation
parameters specify how often to use the learned distribution to generate
grasp plans. A value of 1 means always use random parameter selection, while a
value of 0 means always generate parameters from the current distribution of
the layer. The tag ‘rot’ indicates the Wrist Rotation layer while ‘off’
indicates the Hand Position Layer.
rotRandomness
1 # 1=full random 0=from the
pdf
offRandomness
1 # 1=full random 0=from the
pdf
Now we present the simulation parameters used for learning
to grasp experiments presented in the thesis. The default values of the
parameters given in the descriptions above will not be repeated.
Simulation parameters for
sections 6.6.1 and 6.6.2, and Figure
5.8
hand_rotBANK_code_len
11
hand_rotPITCH_code_len
11
hand_rotHEADING_code_len 6
hand_locMER_code_len
6
hand_locPAR_code_len
6
hand_locRAD_code_len
1
MAXREACH N/A
MAXROTATE 55
MAXBABBLE 50000 (stopped at 16000)
weightSave 16000
Reach2Target MIDDLE0
costThreshold 0.75
palmThreshold 125
negReinforcement -0.1
rotRandomness 0.95
offRandomness 0.95
Simulation parameters for section
5.7 (except Figure
5.8)
hand_rotBANK_code_len
11
hand_rotPITCH_code_len
11
hand_rotHEADING_code_len 6
hand_locMER_code_len
6
hand_locPAR_code_len
6
hand_locRAD_code_len
1
MAXREACH N/A
MAXROTATE 55
MAXBABBLE 50000 (stopped at 10000)
weightSave 5000
Reach2Target INDEX0
costThreshold 0.75
palmThreshold 125
negReinforcement -0.1
rotRandomness 0.95
offRandomness 0.95
Simulation parameters for
sections 5.9 and 6.7
hand_rotBANK_code_len
12
hand_rotPITCH_code_len
7
hand_rotHEADING_code_len 1
hand_locMER_code_len
1
hand_locPAR_code_len
1
hand_locRAD_code_len
1
MAXREACH 1
MAXROTATE 25
MAXBABBLE 200000 (stopped at 20000)
weightSave 2500
Reach2Target MIDDLE0
costThreshold 0.85
palmThreshold 150
negReinforcement -0.1
rotRandomness 1
offRandomness 1
Simulation parameters for
sections 5.8
hand_rotBANK_code_len
9
hand_rotPITCH_code_len
9
hand_rotHEADING_code_len 1
hand_locMER_code_len
7
hand_locPAR_code_len
7
hand_locRAD_code_len
1
MAXREACH 1
MAXROTATE 7
MAXBABBLE 200000 (stopped at 45000)
weightSave 4500
Reach2Target INDEX2
costThreshold 0.80
palmThreshold 150
negReinforcement -0.05
rotRandomness 1
offRandomness 1
Simulation parameters for section
6.9
maxRAD 1200
hand_rotBANK_code_len
10
hand_rotPITCH_code_len
7
hand_rotHEADING_code_len 5
hand_locMER_code_len
10
hand_locPAR_code_len
10
hand_locRAD_code_len
1
MAXREACH 10
MAXROTATE 30
MAXBABBLE 200000 (stopped at 50000)
weightSave 10000
Reach2Target MIDDLE0
costThreshold 0.75
palmThreshold 128
negReinforcement -0.1
rotRandomness 1
offRandomness 1
Simulation parameters for section
6.8
hand_rotBANK_code_len
9
hand_rotPITCH_code_len
9
hand_rotHEADING_code_len 1
hand_locMER_code_len
7
hand_locPAR_code_len
7
hand_locRAD_code_len
1
MAXREACH 10
MAXROTATE 10
MAXBABBLE 200000 (stopped at 50000)
weightSave 4500
Reach2Target INDEX1
costThreshold 0.8
palmThreshold 150
negReinforcement -0.05
rotRandomness 0.95
offRandomness 0.95































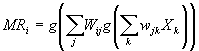
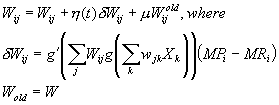

















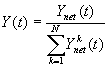
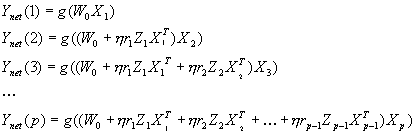



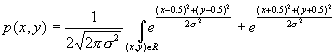 ,
, 
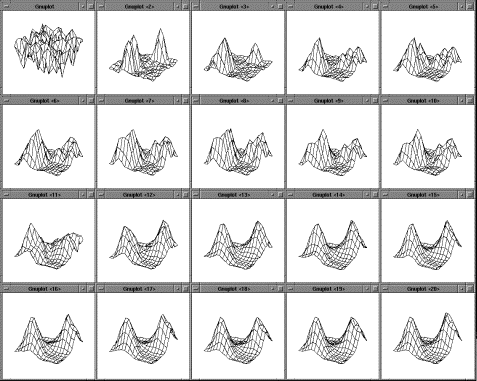

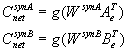
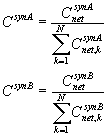
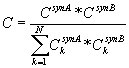
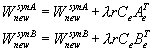 , s
, s